Cancer-selective, single agent chemoradiosensitising gold nanoparticles
- PMID: 28700660
- PMCID: PMC5507319
- DOI: 10.1371/journal.pone.0181103
Cancer-selective, single agent chemoradiosensitising gold nanoparticles
Abstract
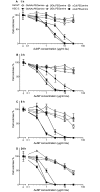
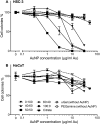
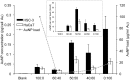
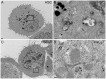
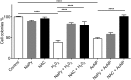
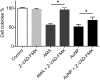
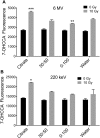
Two nanometre gold nanoparticles (AuNPs), bearing sugar moieties and/or thiol-polyethylene glycol-amine (PEG-amine), were synthesised and evaluated for their in vitro toxicity and ability to radiosensitise cells with 220 kV and 6 MV X-rays, using four cell lines representing normal and cancerous skin and breast tissues. Acute 3 h exposure of cells to AuNPs, bearing PEG-amine only or a 50:50 ratio of alpha-galactose derivative and PEG-amine resulted in selective uptake and toxicity towards cancer cells at unprecedentedly low nanomolar concentrations. Chemotoxicity was prevented by co-administration of N-acetyl cysteine antioxidant, or partially prevented by the caspase inhibitor Z-VAD-FMK. In addition to their intrinsic cancer-selective chemotoxicity, these AuNPs acted as radiosensitisers in combination with 220 kV or 6 MV X-rays. The ability of AuNPs bearing simple ligands to act as cancer-selective chemoradiosensitisers at low concentrations is a novel discovery that holds great promise in developing low-cost cancer nanotherapeutics.
Conflict of interest statement
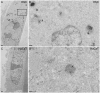
Figures


References
-
- Kwatra D, Venugopal A, Anant S. Nanoparticles in radiation therapy: a summary of various approaches to enhance radiosensitization in cancer. Transl Cancer Res. 2013;2: 330–342.
-
- Pottier A, Borghi E, Levy L. The future of nanosized radiation enhancers. Br J Radiol. British Institute of Radiology; 2015;88: 20150171 doi: 10.1259/bjr.20150171 - DOI - PMC - PubMed
-
- McMahon SJ, Paganetti H, Prise KM. Optimising element choice for nanoparticle radiosensitisers. Nanoscale. 2016; doi: 10.1039/C5NR07089A - DOI - PubMed
-
- McMahon SJ, Hyland WB, Muir MF, Coulter JA, Jain S, Butterworth KT, et al. Biological consequences of nanoscale energy deposition near irradiated heavy atom nanoparticles. Sci Rep. Nature Publishing Group; 2011;1: 18 doi: 10.1038/srep00018 - DOI - PMC - PubMed
-
- Hainfeld JF, Dilmanian FA, Slatkin DN, Smilowitz HM. Radiotherapy enhancement with gold nanoparticles. J Pharm Pharmacol. Blackwell Publishing Ltd; 2008;60: 977–985. doi: 10.1211/jpp.60.8.0005 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

