Improved high sensitivity screen for Huntington disease using a one-step triplet-primed PCR and melting curve assay
- PMID: 28700716
- PMCID: PMC5507316
- DOI: 10.1371/journal.pone.0180984
Improved high sensitivity screen for Huntington disease using a one-step triplet-primed PCR and melting curve assay
Abstract
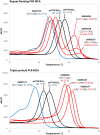
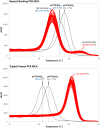
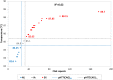
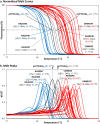
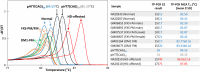
Molecular diagnosis of Huntington disease (HD) is currently performed by fluorescent repeat-flanking or triplet-primed PCR (TP-PCR) with capillary electrophoresis (CE). However, CE requires multiple post-PCR steps and may result in high cost in high-throughput settings. We previously described a cost-effective single-step molecular screening strategy employing the use of melting curve analysis (MCA). However, because it relies on repeat-flanking PCR, its efficiency in detecting expansion mutations decreases with increasing size of the repeat, which could lead to false-negative results. To address this pitfall, we have developed an improved screening assay coupling TP-PCR, which has been shown in CE-based assays to detect all expanded alleles regardless of size, with MCA in a rapid one-step assay. A companion protocol for rapid size confirmation of expansion-positive samples is also described. The assay was optimized on 30 genotype-known DNAs, and two plasmids pHTT(CAG)26 and pHTT(CAG)33 were used to establish the threshold temperatures (TTs) distinguishing normal from expansion-positive samples. In contrast to repeat-flanking PCR MCA, TP-PCR MCA displayed much higher sensitivity for detecting large expansions. All 30 DNAs generated distinct melt peak Tms which correlated well with each sample's larger allele. Normal samples were clearly distinguished from affected samples. The companion sizing protocol accurately sized even the largest expanded allele of ~180 CAGs. Blinded analysis of 69 clinical samples enriched for HD demonstrated 100% assay sensitivity and specificity in sample segregation. The assay targets the HTT CAG repeat specifically, tolerates a wide range of input DNA, and works well using DNA from saliva and buccal swab in addition to blood. Therefore, rapid, accurate, reliable, and high-throughput detection/exclusion of HD can be achieved using this one-step screening assay, at less than half the cost of fluorescent PCR with CE.
Conflict of interest statement
Figures



References
-
- Warby SC, Visscher H, Collins JA, Doty CN, Carter C, Butland SL, et al. HTT haplotypes contribute to differences in Huntington disease prevalence between Europe and East Asia. Eur J Hum Genet. 2011;19(5):561–6. Epub 2011/01/21. doi: 10.1038/ejhg.2010.229 . - DOI - PMC - PubMed
-
- The Huntington's Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72(6):971–83. Epub 1993/03/26. 0092-8674(93)90585-E. . - PubMed
-
- Finkbeiner S. Huntington's Disease. Cold Spring Harb Perspect Biol. 2011;3(a007476). Epub 2011/03/29. doi: 10.1101/cshperspect.a007476 . - DOI - PMC - PubMed
-
- Semaka A, Collins JA, Hayden MR. Unstable familial transmissions of Huntington disease alleles with 27–35 CAG repeats (intermediate alleles). Am J Med Genet B Neuropsychiatr Genet. 2009;153B(1):314–20. Epub 2009/05/21. doi: 10.1002/ajmg.b.30970 . - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

