Endothelial Glycocalyx-Mediated Nitric Oxide Production in Response to Selective AFM Pulling
- PMID: 28700908
- PMCID: PMC5510764
- DOI: 10.1016/j.bpj.2017.05.033
Endothelial Glycocalyx-Mediated Nitric Oxide Production in Response to Selective AFM Pulling
Abstract
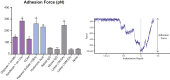
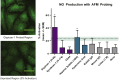
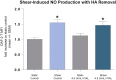
Nitric oxide (NO) is a regulatory molecule in the vascular system and its inhibition due to endothelial injury contributes to cardiovascular disease. The glycocalyx is a thin layer of glycolipids, glycoproteins, and proteoglycans on the surface of mammalian epithelial cells. Extracellular forces are transmitted through the glycocalyx to initiate intracellular signaling pathways. In endothelial cells (ECs), previous studies have shown the glycocalyx to be a significant mediator of NO production; degradation of the endothelial glycocalyx layer (EGL) drastically reduces EC production of NO in response to fluid shear stress. However, the specific EGL components involved in this process are not well established. Recent work using short-hairpin RNA approaches in vitro suggest that the proteoglycan glypican-1, not syndecan-1, is the dominant core protein mediating shear-induced NO production. We utilized atomic force microscopy (AFM) to apply force selectively to components of the EGL of confluent rat fat pad ECs (RFPECs), including proteoglycans and glycosaminoglycans, to observe how each component individually contributes to force-induced production of NO. 4,5-diaminofluorescein diacetate, a cell-permeable fluorescent molecule, was used to detect changes in intracellular NO production. Antibody-coated AFM probes exhibited strong surface binding to RFPEC monolayers, with 100-300 pN mean adhesion forces. AFM pulling on glypican-1 and heparan sulfate for 10 min caused significantly increased NO production, whereas pulling on syndecan-1, CD44, hyaluronic acid, and with control probes did not. We conclude that AFM pulling can be used to activate EGL-mediated NO production and that the heparan sulfate proteoglycan glypican-1 is a primary mechanosensor for shear-induced NO production.
Copyright © 2017 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Shear-induced endothelial NOS activation and remodeling via heparan sulfate, glypican-1, and syndecan-1.Integr Biol (Camb). 2014 Mar;6(3):338-47. doi: 10.1039/c3ib40199e. Epub 2014 Jan 30. Integr Biol (Camb). 2014. PMID: 24480876 Free PMC article.
-
The cancer cell glycocalyx proteoglycan Glypican-1 mediates interstitial flow mechanotransduction to enhance cell migration and metastasis.Biorheology. 2019;56(2-3):151-161. doi: 10.3233/BIR-180203. Biorheology. 2019. PMID: 31256115
-
Heparan sulfate proteoglycan is a mechanosensor on endothelial cells.Circ Res. 2003 Nov 14;93(10):e136-42. doi: 10.1161/01.RES.0000101744.47866.D5. Epub 2003 Oct 16. Circ Res. 2003. PMID: 14563712
-
Endothelial glycocalyx as a critical signalling platform integrating the extracellular haemodynamic forces and chemical signalling.J Cell Mol Med. 2017 Aug;21(8):1457-1462. doi: 10.1111/jcmm.13081. Epub 2017 Feb 17. J Cell Mol Med. 2017. PMID: 28211170 Free PMC article. Review.
-
Mechanotransduction and the endothelial glycocalyx: Interactions with membrane and cytoskeletal proteins to transduce force.Curr Top Membr. 2023;91:43-60. doi: 10.1016/bs.ctm.2023.02.003. Epub 2023 Mar 10. Curr Top Membr. 2023. PMID: 37080680 Review.
Cited by
-
Complement factor H in molecular regulation of angiogenesis.Med Rev (2021). 2024 Jul 1;4(5):452-466. doi: 10.1515/mr-2023-0048. eCollection 2024 Oct. Med Rev (2021). 2024. PMID: 39444793 Free PMC article. Review.
-
Blood Flow Forces in Shaping the Vascular System: A Focus on Endothelial Cell Behavior.Front Physiol. 2020 Jun 5;11:552. doi: 10.3389/fphys.2020.00552. eCollection 2020. Front Physiol. 2020. PMID: 32581842 Free PMC article. Review.
-
On the examination of the viscous response of the brachial artery during flow-mediated dilation.J Mech Behav Biomed Mater. 2022 Jul;131:105255. doi: 10.1016/j.jmbbm.2022.105255. Epub 2022 Apr 27. J Mech Behav Biomed Mater. 2022. PMID: 35500495 Free PMC article.
-
Pro-atherosclerotic disturbed flow disrupts caveolin-1 expression, localization, and function via glycocalyx degradation.J Transl Med. 2018 Dec 18;16(1):364. doi: 10.1186/s12967-018-1721-2. J Transl Med. 2018. PMID: 30563532 Free PMC article.
-
The Structure and Function of the Glycocalyx and Its Connection With Blood-Brain Barrier.Front Cell Neurosci. 2021 Oct 7;15:739699. doi: 10.3389/fncel.2021.739699. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34690703 Free PMC article. Review.
References
-
- van den Berg B.M., Spaan J.A.E., Vink H. Impaired glycocalyx barrier properties contribute to enhanced intimal low-density lipoprotein accumulation at the carotid artery bifurcation in mice. Pflugers Arch. 2009;457:1199–1206. - PubMed
-
- Ignarro L.J. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ. Res. 1989;65:1–21. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

