Dynamic regulation of lysine acetylation: the balance between acetyltransferase and deacetylase activities
- PMID: 28701313
- PMCID: PMC5668585
- DOI: 10.1152/ajprenal.00313.2017
Dynamic regulation of lysine acetylation: the balance between acetyltransferase and deacetylase activities
Abstract
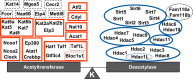
Reversible posttranslational modification of proteins is a critically important process in physiological regulation in all tissues, including the kidney. Lysine acetylation occurs in all organisms, including prokaryotes, and is regulated by a balance between the lysine acetyltransferases (adding an acetyl group to the ε-amino group of a lysine) and deacetylases (removing it). The kidney is an organ rich with acetylated lysines, which map to >2,000 unique histone and nonhistone proteins. However, the functional significance of these modifications remains to be discovered. Here, we have compiled gene lists of the acetyltransferases and deacetylases in the mammalian genomes and mapped their mRNA expression along the renal tubule. These lists will be useful for generating targeted approaches to test the physiological or pathophysiological significance of lysine acetylation changes in the kidney.
Keywords: acetyltransferase; deacetylase; histone; lysine acetylation; nephron.
Figures
References
-
- Belikoff E, Wong LJ, Alberts BM. Extensive purification of histone acetylase A, the major histone N-acetyl transferase activity detected in mammalian cell nuclei. J Biol Chem 255: 11448–11453, 1980. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

