Characterization of Trichome-Expressed BAHD Acyltransferases in Petunia axillaris Reveals Distinct Acylsugar Assembly Mechanisms within the Solanaceae
- PMID: 28701351
- PMCID: PMC5580754
- DOI: 10.1104/pp.17.00538
Characterization of Trichome-Expressed BAHD Acyltransferases in Petunia axillaris Reveals Distinct Acylsugar Assembly Mechanisms within the Solanaceae
Abstract
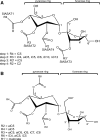
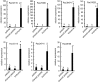
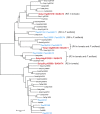
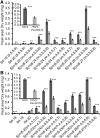
Acylsugars are synthesized in the glandular trichomes of the Solanaceae family and are implicated in protection against abiotic and biotic stress. Acylsugars are composed of either sucrose or glucose esterified with varying numbers of acyl chains of differing length. In tomato (Solanum lycopersicum), acylsugar assembly requires four acylsugar acyltransferases (ASATs) of the BAHD superfamily. Tomato ASATs catalyze the sequential esterification of acyl-coenzyme A thioesters to the R4, R3, R3', and R2 positions of sucrose, yielding a tetra-acylsucrose. Petunia spp. synthesize acylsugars that are structurally distinct from those of tomato. To explore the mechanisms underlying this chemical diversity, a Petuniaaxillaris transcriptome was mined for trichome preferentially expressed BAHDs. A combination of phylogenetic analyses, gene silencing, and biochemical analyses coupled with structural elucidation of metabolites revealed that acylsugar assembly is not conserved between tomato and petunia. In P. axillaris, tetra-acylsucrose assembly occurs through the action of four ASATs, which catalyze sequential addition of acyl groups to the R2, R4, R3, and R6 positions. Notably, in P. axillaris, PaxASAT1 and PaxASAT4 catalyze the acylation of the R2 and R6 positions of sucrose, respectively, and no clear orthologs exist in tomato. Similarly, petunia acylsugars lack an acyl group at the R3' position, and congruently, an ortholog of SlASAT3, which catalyzes acylation at the R3' position in tomato, is absent in P. axillaris Furthermore, where putative orthologous relationships of ASATs are predicted between tomato and petunia, these are not supported by biochemical assays. Overall, these data demonstrate the considerable evolutionary plasticity of acylsugar biosynthesis.
© 2017 American Society of Plant Biologists. All Rights Reserved.
Figures



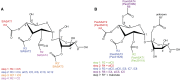
References
-
- Arimura G, Matsui K, Takabayashi J (2009) Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant Cell Physiol 50: 911–923 - PubMed
-
- Arrendale RF, Severson RF, Sisson VA, Costello CE, Leary JA, Himmelsbach DS, Vanhalbeek H (1990) Characterization of the sucrose ester fraction from Nicotiana glutinosa. J Agric Food Chem 38: 75–85
-
- Bedewitz MA, Góngora-Castillo E, Uebler JB, Gonzales-Vigil E, Wiegert-Rininger KE, Childs KL, Hamilton JP, Vaillancourt B, Yeo YS, Chappell J, et al. (2014) A root-expressed L-phenylalanine:4-hydroxyphenylpyruvate aminotransferase is required for tropane alkaloid biosynthesis in Atropa belladonna. Plant Cell 26: 3745–3762 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

