Mechanism of action of the anti-inflammatory connexin43 mimetic peptide JM2
- PMID: 28701358
- PMCID: PMC5625091
- DOI: 10.1152/ajpcell.00229.2016
Mechanism of action of the anti-inflammatory connexin43 mimetic peptide JM2
Abstract
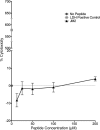
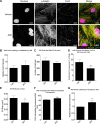
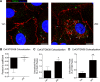
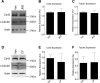
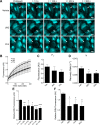
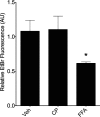
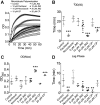
Connexin-based therapeutics have shown the potential for therapeutic efficacy in improving wound healing. Our previous work demonstrated that the connexin43 (Cx43) mimetic peptide juxtamembrane 2 (JM2) reduced the acute inflammatory response to a submuscular implant model by inhibiting purinergic signaling. Given the prospective application in improving tissue-engineered construct tolerance that these results indicated, we sought to determine the mechanism of action for JM2 in the present study. Using confocal microscopy, a gap-FRAP cell communication assay, and an ethidium bromide uptake assay of hemichannel function we found that the peptide reduced cell surface Cx43 levels, Cx43 gap junction (GJ) size, GJ communication, and hemichannel activity. JM2 is based on the sequence of the Cx43 microtubule binding domain, and microtubules have a confirmed role in intracellular trafficking of Cx43 vesicles. Therefore, we tested the effect of JM2 on Cx43-microtubule interaction and microtubule polymerization. We found that JM2 enhanced Cx43-microtubule interaction and that microtubule polymerization was significantly enhanced. Taken together, these data suggest that JM2 inhibits trafficking of Cx43 to the cell surface by promoting irrelevant microtubule polymerization and thereby reduces the number of hemichannels in the plasma membrane available to participate in proinflammatory purinergic signaling. Importantly, this work indicates that JM2 may have therapeutic value in the treatment of proliferative diseases such as cancer. We conclude that the targeted action of JM2 on Cx43 channels may improve the tolerance of implanted tissue-engineered constructs against the innate inflammatory response.
Keywords: cancer; connexin43; gap junction; hemichannel; inflammation.
Copyright © 2017 the American Physiological Society.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

