The Cross-Species Mycobacterial Growth Inhibition Assay (MGIA) Project, 2010-2014
- PMID: 28701467
- PMCID: PMC5585695
- DOI: 10.1128/CVI.00142-17
The Cross-Species Mycobacterial Growth Inhibition Assay (MGIA) Project, 2010-2014
Abstract
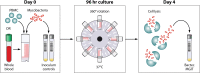
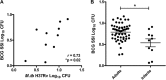
The development of a functional biomarker assay in the tuberculosis (TB) field would be widely recognized as a major advance in efforts to develop and to test novel TB vaccine candidates efficiently. We present preliminary studies using mycobacterial growth inhibition assays (MGIAs) to detect Mycobacterium bovis BCG vaccine responses across species, and we extend this work to determine whether a standardized MGIA can be applied in characterizing new TB vaccines. The comparative MGIA studies reviewed here aimed to evaluate robustness, reproducibility, and ability to reflect in vivo responses. In doing so, they have laid the foundation for the development of a MGIA that can be standardized and potentially qualified. A major challenge ahead lies in better understanding the relationships between in vivo protection, in vitro growth inhibition, and the immune mechanisms involved. The final outcome would be a MGIA that could be used with confidence in TB vaccine trials. We summarize data arising from this project, present a strategy to meet the goals of developing a functional assay for TB vaccine testing, and describe some of the challenges encountered in performing and transferring such assays.
Keywords: MGIA; correlates of immunity; mycobacterial growth inhibition assay; tuberculosis; vaccines.
Copyright © 2017 Brennan et al.
Figures
















References
-
- Fletcher HA, Snowden MA, Landry B, Rida W, Satti I, Harris SA, Matsumiya M, Tanner R, O'Shea MK, Dheenadhayalan V, Bogardus L, Stockdale L, Marsay L, Chomka A, Harrington-Kandt R, Manjaly-Thomas ZR, Naranbhai V, Stylianou E, Darboe F, Penn-Nicholson A, Nemes E, Hatheril M, Hussey G, Mahomed H, Tameris M, McClain JB, Evans TG, Hanekom WA, Scriba TJ, McShane H. 2016. T-cell activation is an immune correlate of risk in BCG vaccinated infants. Nat Commun 7:11290. doi:10.1038/ncomms11290. - DOI - PMC - PubMed
-
- Fletcher HA, Filali-Mouhim A, Nemes E, Hawkridge A, Keyser A, Njikan S, Hatherill M, Scriba TJ, Abel B, Kagina BM, Veldsman A, Agudelo NM, Kaplan G, Hussey GD, Sekaly RP, Hanekom WA. 2016. Human newborn bacille Calmette-Guérin vaccination and risk of tuberculosis disease: a case-control study. BMC Med 14:76. doi:10.1186/s12916-016-0617-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

