Rubella Surveillance and Diagnostic Testing among a Low-Prevalence Population, New York City, 2012-2013
- PMID: 28701468
- PMCID: PMC5585696
- DOI: 10.1128/CVI.00102-17
Rubella Surveillance and Diagnostic Testing among a Low-Prevalence Population, New York City, 2012-2013
Abstract
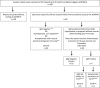
The New York City Department of Health and Mental Hygiene (DOHMH) receives clinical and laboratory reports for rubella. Because rubella immunoglobulin M (IgM) assays may produce false-positive results and rubella infections may be asymptomatic, interpretation of positive IgM results can be challenging. Rubella reports received by DOHMH in 2012 to 2013 were reviewed. The rubella IgM testing purpose was determined through case investigation. Results of IgM testing by indirect enzyme-linked immunosorbent assay (ELISA) and capture enzyme immunoassay (EIA) were compared to determine positive predictive value (PPV) and specificity. DOHMH received 199 rubella reports; 2 were true cases. Of all reports, 77.9% were tested for rubella IgM erroneously, 19.6% were tested for diagnostic purposes, 2.0% had unknown test purpose, and 0.5% were not tested. PPV of indirect ELISA was 6% overall, 14% for diagnostic tests, and 0% for tests ordered erroneously. PPV of capture EIA was 29% overall, 50% for diagnostic tests, and 0% for tests ordered erroneously. Overall, specificity was 52% for indirect ELISA and 85% for capture EIA. Limiting rubella IgM testing to patients for whom rubella diagnosis is suspected and using a more specific IgM assay have the potential to reduce false-positive rubella IgM results.
Keywords: immunoassays; immunoglobulin M; rubella; serology; surveillance.
Copyright © 2017 American Society for Microbiology.
Figures
References
-
- Centers for Disease Control and Prevention. 2005. Elimination of rubella and congenital rubella syndrome—United States, 1969–2004. MMWR Morb Mortal Wkly Rep 54:279–282. - PubMed
-
- Pan American Health Organization. 2015. Americas region is declared the world's first to eliminate rubella. http://www.paho.org/Hq/index.php?option=com_content&view=article&id=1079... Accessed 23 March 2017.
-
- Centers for Disease Control and Prevention. 2012. Manual for the surveillance of vaccine-preventable diseases. Centers for Disease Control and Prevention, Atlanta, GA.
-
- Anonymous. 1993. Immunization during pregnancy. ACOG technical bulletin number 160—October 1991. Int J Gynaecol Obstet 40:69–79. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

