Effect of Ferric Carboxymaltose on Exercise Capacity in Patients With Chronic Heart Failure and Iron Deficiency
- PMID: 28701470
- PMCID: PMC5642327
- DOI: 10.1161/CIRCULATIONAHA.117.027497
Effect of Ferric Carboxymaltose on Exercise Capacity in Patients With Chronic Heart Failure and Iron Deficiency
Abstract
Background: Iron deficiency is common in patients with heart failure (HF) and is associated with reduced exercise capacity and poor outcomes. Whether correction of iron deficiency with (intravenous) ferric carboxymaltose (FCM) affects peak oxygen consumption [peak VO2], an objective measure of exercise intolerance in HF, has not been examined.
Methods: We studied patients with systolic HF (left ventricular ejection fraction ≤45%) and mild to moderate symptoms despite optimal HF medication. Patients were randomized 1:1 to treatment with FCM for 24 weeks or standard of care. The primary end point was the change in peak VO2 from baseline to 24 weeks. Secondary end points included the effect on hematinic and cardiac biomarkers, quality of life, and safety. For the primary analysis, patients who died had a value of 0 imputed for 24-week peak VO2. Additional sensitivity analyses were performed to determine the impact of imputation of missing peak VO2 data.
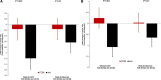
Results: A total of 172 patients with HF were studied and received FCM (n=86) or standard of care (control group, n=86). At baseline, the groups were well matched; mean age was 64 years, 75% were male, mean left ventricular ejection fraction was 32%, and peak VO2 was 13.5 mL/min/kg. FCM significantly increased serum ferritin and transferrin saturation. At 24 weeks, peak VO2 had decreased in the control group (least square means -1.19±0.389 mL/min/kg) but was maintained on FCM (-0.16±0.387 mL/min/kg; P=0.020 between groups). In a sensitivity analysis, in which missing data were not imputed, peak VO2 at 24 weeks decreased by -0.63±0.375 mL/min/kg in the control group and by -0.16±0.373 mL/min/kg in the FCM group; P=0.23 between groups). Patients' global assessment and functional class as assessed by the New York Heart Association improved on FCM versus standard of care.
Conclusions: Treatment with intravenous FCM in patients with HF and iron deficiency improves iron stores. Although a favorable effect on peak VO2 was observed on FCM, compared with standard of care in the primary analysis, this effect was highly sensitive to the imputation strategy for peak VO2 among patients who died. Whether FCM is associated with an improved outcome in these high-risk patients needs further study.
Clinical trial registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT01394562.
Keywords: exercise capacity; ferric carboxymaltose; heart failure; iron deficiency.
© 2017 The Authors.
Figures

Comment in
-
"Pumping Iron" to Improve Exercise Performance in Heart Failure: New Data and New Guidelines.Circulation. 2017 Oct 10;136(15):1384-1386. doi: 10.1161/CIRCULATIONAHA.117.030530. Circulation. 2017. PMID: 28993371 No abstract available.
References
-
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. - PubMed
-
- Van Veldhuisen DJ, Anker SD, Ponikowski P, Macdougall IC. Anaemia and iron deficiency in heart failure: mechanisms and therapeutic applications. Nat Rev Cardiol. 2011;8:485–493. doi: 10.1038/nrcardio.2011.77. - PubMed
-
- Klip IT, Comin-Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, Lok DJ, Rosentryt P, Torrens A, Polonski L, van Veldhuisen DJ, van der Meer P, Jankowska EA. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J. 2013;165:575.e3–582.e3. doi: 10.1016/j.ahj.2013.01.017. - PubMed
-
- Okonko DO, Mandal AK, Missouris CG, Poole-Wilson PA. Disordered iron homeostasis in chronic heart failure: prevalence, predictors, and relation to anemia, exercise capacity, and survival. J Am Coll Cardiol. 2011;58:1241–1251. doi: 10.1016/j.jacc.2011.04.040. - PubMed
-
- Cleland JG, Zhang J, Pellicori P, Dicken B, Dierckx R, Shoaib A, Wong K, Rigby A, Goode K, Clark AL. Prevalence and outcomes of anemia and hematinic deficiencies in patients with chronic heart failure. JAMA Cardiol. 2016;1:539–547. doi: 10.1001/jamacardio.2016.1161. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

