Detection of early pancreatic ductal adenocarcinoma with thrombospondin-2 and CA19-9 blood markers
- PMID: 28701476
- PMCID: PMC5727893
- DOI: 10.1126/scitranslmed.aah5583
Detection of early pancreatic ductal adenocarcinoma with thrombospondin-2 and CA19-9 blood markers
Abstract
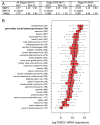
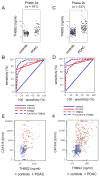
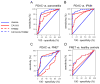
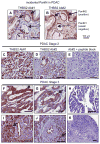
Markers are needed to facilitate early detection of pancreatic ductal adenocarcinoma (PDAC), which is often diagnosed too late for effective therapy. Starting with a PDAC cell reprogramming model that recapitulated the progression of human PDAC, we identified secreted proteins and tested a subset as potential markers of PDAC. We optimized an enzyme-linked immunosorbent assay (ELISA) using plasma samples from patients with various stages of PDAC, from individuals with benign pancreatic disease, and from healthy controls. A phase 1 discovery study (n = 20), a phase 2a validation study (n = 189), and a second phase 2b validation study (n = 537) revealed that concentrations of plasma thrombospondin-2 (THBS2) discriminated among all stages of PDAC consistently. The receiver operating characteristic (ROC) c-statistic was 0.76 in the phase 1 study, 0.84 in the phase 2a study, and 0.87 in the phase 2b study. The plasma concentration of THBS2 was able to discriminate resectable stage I cancer as readily as stage III/IV PDAC tumors. THBS2 plasma concentrations combined with those for CA19-9, a previously identified PDAC marker, yielded a c-statistic of 0.96 in the phase 2a study and 0.97 in the phase 2b study. THBS2 data improved the ability of CA19-9 to distinguish PDAC from pancreatitis. With a specificity of 98%, the combination of THBS2 and CA19-9 yielded a sensitivity of 87% for PDAC in the phase 2b study. A THBS2 and CA19-9 blood marker panel measured with a conventional ELISA may improve the detection of patients at high risk for PDAC.
Copyright © 2017 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures
Comment in
-
Pancreatic cancer: Biomarkers for the early detection of PDAC.Nat Rev Gastroenterol Hepatol. 2017 Sep;14(9):504-505. doi: 10.1038/nrgastro.2017.111. Epub 2017 Aug 2. Nat Rev Gastroenterol Hepatol. 2017. PMID: 28765582 No abstract available.
References
-
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. - PubMed
-
- Chari ST, Kelly K, Hollingsworth MA, Thayer SP, Ahlquist DA, Andersen DK, Batra SK, Brentnall TA, Canto M, Cleeter DF, Firpo MA, Gambhir SS, Go VL, Hines OJ, Kenner BJ, Klimstra DS, Lerch MM, Levy MJ, Maitra A, Mulvihill SJ, Petersen GM, Rhim AD, Simeone DM, Srivastava S, Tanaka M, Vinik AI, Wong D. Early detection of sporadic pancreatic cancer: summative review. Pancreas. 2015;44:693–712. - PMC - PubMed
-
- Satake K, Kanazawa G, Kho I, Chung YS, Umeyama K. A clinical evaluation of carbohydrate antigen 19-9 and carcinoembryonic antigen in patients with pancreatic carcinoma. J Surg Oncol. 1985;29:15–21. - PubMed
-
- Duffy MJ, Sturgeon C, Lamerz R, Haglund C, Holubec VL, Klapdor R, Nicolini A, Topolcan O, Heinemann V. Tumor markers in pancreatic cancer: a European Group on Tumor Markers (EGTM) status report. Ann Oncol. 2010;21:441–447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

