The CREST-E study of creatine for Huntington disease: A randomized controlled trial
- PMID: 28701493
- PMCID: PMC5562960
- DOI: 10.1212/WNL.0000000000004209
The CREST-E study of creatine for Huntington disease: A randomized controlled trial
Abstract
Objective: To investigate whether creatine administration could slow progressive functional decline in adults with early symptoms of Huntington disease.
Methods: We conducted a multicenter, randomized, double-blind, placebo-controlled study of up to 40 g daily of creatine monohydrate in participants with stage I and II HD treated for up to 48 months. The primary outcome measure was the rate of change in total functional capacity (TFC) between baseline and end of follow-up. Secondary outcome measures included changes in additional clinical scores, tolerability, and quality of life. Safety was assessed by adverse events and laboratory studies.
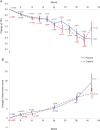
Results: At 46 sites in North America, Australia, and New Zealand, 553 participants were randomized to creatine (275) or placebo (278). The trial was designed to enroll 650 patients, but was halted for futility after the first interim analysis. The estimated rates of decline in the primary outcome measure (TFC) were 0.82 points per year for participants on creatine, 0.70 points per year for participants on placebo, favoring placebo (nominal 95% confidence limits -0.11 to 0.35). Adverse events, mainly gastrointestinal, were significantly more common in participants on creatine. Serious adverse events, including deaths, were more frequent in the placebo group. Subgroup analysis suggested that men and women may respond differently to creatine treatment.
Conclusions: Our data do not support the use of creatine treatment for delaying functional decline in early manifest HD.
Clinicaltrialsgov identifier: NCT00712426.
Classification of evidence: This study provides Class II evidence that for patients with early symptomatic HD, creatine monohydrate is not beneficial for slowing functional decline.
Copyright © 2017 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
References
-
- Ross CA, Aylward EH, Wild EJ, et al. . Huntington disease: natural history, biomarkers and prospects for therapeutics. Nat Rev Neurol 2014;10:204–216. - PubMed
-
- Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR. Functions and effects of creatine in the central nervous system. Brain Res Bull 2008;76:329–343. - PubMed
-
- Andreassen OA, Dedeoglu A, Ferrante RJ, et al. . Creatine increases survival and delays motor symptoms in a transgenic animal model of Huntington's disease. Neurobiol Dis 2001;8:479–491. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
