Blocking TGF- β and β-Catenin Epithelial Crosstalk Exacerbates CKD
- PMID: 28701516
- PMCID: PMC5698068
- DOI: 10.1681/ASN.2016121351
Blocking TGF- β and β-Catenin Epithelial Crosstalk Exacerbates CKD
Abstract
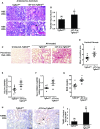
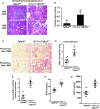
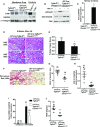
The TGF-β and Wnt/β-catenin pathways have important roles in modulating CKD, but how these growth factors affect the epithelial response to CKD is not well studied. TGF-β has strong profibrotic effects, but this pleiotropic factor has many different cellular effects depending on the target cell type. To investigate how TGF-β signaling in the proximal tubule, a key target and mediator of CKD, alters the response to CKD, we injured mice lacking the TGF-β type 2 receptor specifically in this epithelial segment. Compared with littermate controls, mice lacking the proximal tubular TGF-β receptor had significantly increased tubular injury and tubulointerstitial fibrosis in two different models of CKD. RNA sequencing indicated that deleting the TGF-β receptor in proximal tubule cells modulated many growth factor pathways, but Wnt/β-catenin signaling was the pathway most affected. We validated that deleting the proximal tubular TGF-β receptor impaired β-catenin activity in vitro and in vivo Genetically restoring β-catenin activity in proximal tubules lacking the TGF-β receptor dramatically improved the tubular response to CKD in mice. Deleting the TGF-β receptor alters many growth factors, and therefore, this ameliorated response may be a direct effect of β-catenin activity or an indirect effect of β-catenin interacting with other growth factors. In conclusion, blocking TGF-β and β-catenin crosstalk in proximal tubules exacerbates tubular injury in two models of CKD.
Keywords: chronic kidney disease; fibrosis; proximal tubule; renal epithelial cell.
Copyright © 2017 by the American Society of Nephrology.
Figures



Comment in
-
Surprising Enhancement of Fibrosis by Tubule-Specific Deletion of the TGF-β Receptor: A New Twist on an Old Paradigm.J Am Soc Nephrol. 2017 Dec;28(12):3427-3429. doi: 10.1681/ASN.2017080947. Epub 2017 Oct 26. J Am Soc Nephrol. 2017. PMID: 29074738 Free PMC article. No abstract available.
References
-
- Wrana JL, Attisano L, Cárcamo J, Zentella A, Doody J, Laiho M, Wang XF, Massagué J: TGF beta signals through a heteromeric protein kinase receptor complex. Cell 71: 1003–1014, 1992 - PubMed
-
- Yamashita H, ten Dijke P, Franzén P, Miyazono K, Heldin CH: Formation of hetero-oligomeric complexes of type I and type II receptors for transforming growth factor-beta. J Biol Chem 269: 20172–20178, 1994 - PubMed
-
- Hayashida T, Decaestecker M, Schnaper HW: Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-beta-dependent responses in human mesangial cells. FASEB J 17: 1576–1578, 2003 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

