Measuring and quantifying skin sympathetic nervous system activity in humans
- PMID: 28701539
- PMCID: PMC5626908
- DOI: 10.1152/jn.00283.2017
Measuring and quantifying skin sympathetic nervous system activity in humans
Abstract
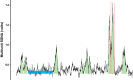
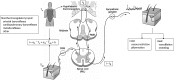
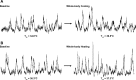
Development of the technique of microneurography has substantially increased our understanding of the function of the sympathetic nervous system (SNS) in health and in disease. The ability to directly record signals from peripheral autonomic nerves in conscious humans allows for qualitative and quantitative characterization of SNS responses to specific stimuli and over time. Furthermore, distinct neural outflow to muscle (MSNA) and skin (SSNA) can be delineated. However, there are limitations and caveats to the use of microneurography, measurement criteria, and signal analysis and interpretation. MSNA recordings have a longer history and are considered relatively more straightforward from a measurement and analysis perspective. This brief review provides an overview of the development of the technique as used to measure SSNA. The focus is on the utility of measuring sympathetic activity directed to the skin, the unique issues related to analyzing and quantifying multiunit SSNA, and the challenges related to its interpretation.
Keywords: MSNA; SSNA; cutaneous blood flow; microneurography; thermoregulation; vasoconstriction; vasodilation.
Copyright © 2017 the American Physiological Society.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

