The MLL recombinome of acute leukemias in 2017
- PMID: 28701730
- PMCID: PMC5808070
- DOI: 10.1038/leu.2017.213
The MLL recombinome of acute leukemias in 2017
Abstract
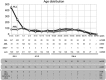
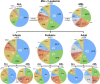
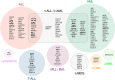
Chromosomal rearrangements of the human MLL/KMT2A gene are associated with infant, pediatric, adult and therapy-induced acute leukemias. Here we present the data obtained from 2345 acute leukemia patients. Genomic breakpoints within the MLL gene and the involved translocation partner genes (TPGs) were determined and 11 novel TPGs were identified. Thus, a total of 135 different MLL rearrangements have been identified so far, of which 94 TPGs are now characterized at the molecular level. In all, 35 out of these 94 TPGs occur recurrently, but only 9 specific gene fusions account for more than 90% of all illegitimate recombinations of the MLL gene. We observed an age-dependent breakpoint shift with breakpoints localizing within MLL intron 11 associated with acute lymphoblastic leukemia and younger patients, while breakpoints in MLL intron 9 predominate in AML or older patients. The molecular characterization of MLL breakpoints suggests different etiologies in the different age groups and allows the correlation of functional domains of the MLL gene with clinical outcome. This study provides a comprehensive analysis of the MLL recombinome in acute leukemia and demonstrates that the establishment of patient-specific chromosomal fusion sites allows the design of specific PCR primers for minimal residual disease analyses for all patients.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Pui CH, Gaynon PS, Boyett JM, Chessells JM, Baruchel A, Kamps W et al. Outcome of treatment in childhood acute lymphoblastic leukaemia with rearrangements of the 11q23 chromosomal region. Lancet 2002; 359: 1909–1915. - PubMed
-
- Pui CH, Chessells JM, Camitta B, Baruchel A, Biondi A, Boyett JM et al. Clinical heterogeneity in childhood acute lymphoblastic leukemia with 11q23 rearrangements. Leukemia 2003; 17: 700–706. - PubMed
-
- Szczepański T, Harrison CJ, van Dongen JJ. Genetic aberrations in paediatric acute leukaemias and implications for management of patients. Lancet Oncol 2010; 11: 880–889. - PubMed
-
- Burmeister T, Marschalek R, Schneider B, Meyer C, Gökbuget N, Schwartz S et al. Monitoring minimal residual disease by quantification of genomic chromosomal breakpoint sequences in acute leukemias with MLL aberrations. Leukemia 2006; 20: 451–457. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

