Secretome of in vitro cultured human embryos contains extracellular vesicles that are uptaken by the maternal side
- PMID: 28701751
- PMCID: PMC5507879
- DOI: 10.1038/s41598-017-05549-w
Secretome of in vitro cultured human embryos contains extracellular vesicles that are uptaken by the maternal side
Abstract
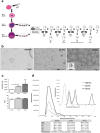
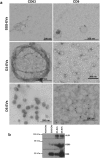
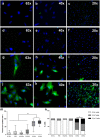
Communication between embryo and maternal endometrium occurs during a specific time frame in which implantation is possible. Here we demonstrate for the first time that conditioned media from non-manipulated human embryos cultured in vitro for 3 days or up to the blastocyst stage contain extracellular vesicles (EVs) with a diameter of 50 to 200 nm and bearing the traditional microvesicle and exosome marker proteins CD63, CD9 and ALIX. The embryonic origin of these EVs has been confirmed by the presence of stemness gene transcripts and their enrichment in the non-classical HLA-G protein. NANOG and POU5F1 transcripts were shown to be contained in vesicles deriving from embryos at different stages of development. In line with a higher detection rate of the HLA-G protein in blastocysts compared to cleavage stage embryos, a significantly higher amount of HLA-G was found in vesicles accumulated in spent media from day 3 to day 5 of development compared to those isolated from the earlier stage. Uptake of dye-labeled embryo-derived EVs by human primary endometrial epithelial and stromal cells was also demonstrated with a fluorescence intensity signal significantly higher for cells treated with vesicles derived from blastocysts. Based on these findings, EV exchange may be suggested as an emerging way of communication at the maternal-fetal interface.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

