Oncolytic measles virus enhances antitumour responses of adoptive CD8+NKG2D+ cells in hepatocellular carcinoma treatment
- PMID: 28701757
- PMCID: PMC5507973
- DOI: 10.1038/s41598-017-05500-z
Oncolytic measles virus enhances antitumour responses of adoptive CD8+NKG2D+ cells in hepatocellular carcinoma treatment
Abstract
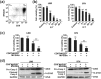
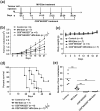
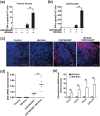
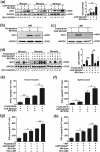
There is an urgent need for novel effective treatment for hepatocellular carcinoma (HCC). Oncolytic viruses (OVs) not only directly lyse malignant cells, but also induce potent antitumour immune responses. The potency and precise mechanisms of antitumour immune activation by attenuated measles virus remain unclear. In this study, we investigated the potency of the measles virus vaccine strain Edmonston (MV-Edm) in improving adoptive CD8+NKG2D+ cells for HCC treatment. We show that MV-Edm-infected HCC enhanced the antitumour activity of CD8+NKG2D+ cells, mediated by at least three distinct mechanisms. First, MV-Edm infection compelled HCC cells to express the specific NKG2D ligands MICA/B, which may contribute to the activation of CD8+NKG2D+ cells. Second, MV-Edm-infected HCC cells stimulated CD8+NKG2D+ cells to express high level of FasL resulting in enhanced induction of apoptosis. Third, intratumoural administration of MV-Edm enhanced infiltration of intravenously injected CD8+NKG2D+ cells. Moreover, we found that MV-Edm and adoptive CD8+NKG2D+ cells, either administered alone or combined, upregulated the immune suppressive enzyme indoleamine 2,3-dioxygenase 1 (IDO1) in HCC. Elimination of IDO1 by fludarabine enhanced antitumour responses. Taken together, our data provide a novel and clinically relevant strategy for treatment of HCC.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Engineered measles virus as a novel oncolytic viral therapy system for hepatocellular carcinoma.Hepatology. 2006 Dec;44(6):1465-77. doi: 10.1002/hep.21437. Hepatology. 2006. PMID: 17133484
-
Recombinant adenovirus expressing the fusion protein PD1PVR improves CD8+ T cell-mediated antitumor efficacy with long-term tumor-specific immune surveillance in hepatocellular carcinoma.Cell Oncol (Dordr). 2021 Dec;44(6):1243-1255. doi: 10.1007/s13402-021-00633-w. Epub 2021 Sep 7. Cell Oncol (Dordr). 2021. PMID: 34491549
-
A bispecific protein rG7S-MICA recruits natural killer cells and enhances NKG2D-mediated immunosurveillance against hepatocellular carcinoma.Cancer Lett. 2016 Mar 28;372(2):166-78. doi: 10.1016/j.canlet.2016.01.001. Epub 2016 Jan 11. Cancer Lett. 2016. PMID: 26791237
-
[Perspectives on immunotherapy for hepatocellular carcinoma].Dtsch Med Wochenschr. 2013 Apr;138(14):740-4. doi: 10.1055/s-0032-1333030. Epub 2013 Mar 26. Dtsch Med Wochenschr. 2013. PMID: 23533044 Review. German.
-
Oncolytic measles viruses for cancer therapy.Expert Opin Biol Ther. 2004 Oct;4(10):1685-92. doi: 10.1517/14712598.4.10.1685. Expert Opin Biol Ther. 2004. PMID: 15461580 Review.
Cited by
-
Oncolytic viral vectors in the era of diversified cancer therapy: from preclinical to clinical.Clin Transl Oncol. 2022 Sep;24(9):1682-1701. doi: 10.1007/s12094-022-02830-x. Epub 2022 May 25. Clin Transl Oncol. 2022. PMID: 35612653 Free PMC article. Review.
-
Measles virus: Background and oncolytic virotherapy.Biochem Biophys Rep. 2018 Jan 2;13:58-62. doi: 10.1016/j.bbrep.2017.12.004. eCollection 2018 Mar. Biochem Biophys Rep. 2018. PMID: 29326986 Free PMC article. Review.
-
Potential of oncolytic viruses in the treatment of multiple myeloma.Oncolytic Virother. 2018 Feb 23;7:1-12. doi: 10.2147/OV.S136644. eCollection 2017. Oncolytic Virother. 2018. PMID: 29503813 Free PMC article. Review.
-
Immunogenic cell death: The cornerstone of oncolytic viro-immunotherapy.Front Immunol. 2023 Jan 23;13:1038226. doi: 10.3389/fimmu.2022.1038226. eCollection 2022. Front Immunol. 2023. PMID: 36755812 Free PMC article. Review.
-
Combination of Oncolytic Measles Virus Armed With BNiP3, a Pro-apoptotic Gene and Paclitaxel Induces Breast Cancer Cell Death.Front Oncol. 2019 Jan 15;8:676. doi: 10.3389/fonc.2018.00676. eCollection 2018. Front Oncol. 2019. PMID: 30697531 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

