Protective effect of ginsenosides Rk3 and Rh4 on cisplatin-induced acute kidney injury in vitro and in vivo
- PMID: 28701862
- PMCID: PMC5489750
- DOI: 10.1016/j.jgr.2016.03.008
Protective effect of ginsenosides Rk3 and Rh4 on cisplatin-induced acute kidney injury in vitro and in vivo
Abstract
Background: Nephrotoxicity is the major side effect in cisplatin chemotherapy. Previously, we reported that the ginsenosides Rk3 and Rh4 reduced cisplatin toxicity on porcine renal proximal epithelial tubular cells (LLC-PK1). Here, we aimed to evaluate the protective effect of ginsenosides Rk3 and Rh4 on kidney function and elucidate their antioxidant effect using in vitro and in vivo models of cisplatin-induced acute renal failure.
Methods: An enriched mixture of ginsenosides Rk3 and Rh4 (KG-KH; 49.3% and 43.1%, respectively) was purified from sun ginseng (heat processed Panax ginseng). Cytotoxicity was induced by treatment of 20μM cisplatin to LLC-PK1 cells and rat model of acute renal failure was generated by single intraperitoneal injection of 5 mg/kg cisplatin. Protective effects were assessed by determining cell viability, reactive oxygen species generation, blood urea nitrogen, serum creatinine, antioxidant enzyme activity, and histopathological examination.
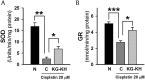
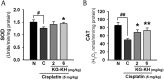
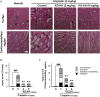
Results: The in vitro assay demonstrated that KG-KH (50 μg/mL) significantly increased cell viability (4.6-fold), superoxide dismutase activity (2.8-fold), and glutathione reductase activity (1.5-fold), but reduced reactive oxygen species generation (56%) compared to cisplatin control cells. KG-KH (6 mg/kg, per os) also significantly inhibited renal edema (87% kidney index) and dysfunction (71.4% blood urea nitrogen, 67.4% creatinine) compared to cisplatin control rats. Of note, KG-KH significantly recovered the kidney levels of catalase (1.2-fold) and superoxide dismutase (1.5-fold).
Conclusion: Considering the oxidative injury as an early trigger of cisplatin nephrotoxicity, our findings suggest that ginsenosides Rk3 and Rh4 protect the kidney from cisplatin-induced oxidative injury and help to recover renal function by restoring intrinsic antioxidant defenses.
Keywords: Panax ginseng; acute kidney injury; cisplatin; ginsenoside Rh4; ginsenoside Rk3.
Figures


References
-
- Lebwohl D., Canetta R. Clinical development of platinum complexes in cancer therapy: an historical perspective and an update. Eur J Cancer. 1998;34:1522–1534. - PubMed
-
- Arany I., Safirstein R.L. Cisplatin nephrotoxicity. Semin Nephrol. 2003;23:460–464. - PubMed
-
- Pabla N., Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. - PubMed
-
- Wang D., Lippard S.J. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–320. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

