Total Synthesis of Viniferifuran, Resveratrol-Piceatannol Hybrid, Anigopreissin A and Analogues - Investigation of Demethylation Strategies
- PMID: 28701908
- PMCID: PMC5484382
- DOI: 10.1002/adsc.201601089
Total Synthesis of Viniferifuran, Resveratrol-Piceatannol Hybrid, Anigopreissin A and Analogues - Investigation of Demethylation Strategies
Abstract
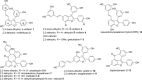
Resveratrol-based natural products constitute a valuable source of unique compounds with diverse biological activities. In this report we investigate demethylation strategies to minimize formation of cyclized and dimerized products during the synthesis of viniferifuran and analogues. We found that boron trichloride/tetra-n-butylammonium iodide (BCl3/TBAI) is typically more effective than boron tribromide (BBr3). Based on these findings we carried out the first syntheses of dehydro-δ-viniferin, resveratrol-piceatannol hybrid and anigopreissin A. In addition, we have developed a short and efficient route to viniferifuran that was obtained in 13% yield over six steps.
Keywords: demethylation; natural products; polyphenols; resveratrol oligomers; stilbenoids; total synthesis.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
