Distinct Features of Doublecortin as a Marker of Neuronal Migration and Its Implications in Cancer Cell Mobility
- PMID: 28701917
- PMCID: PMC5487455
- DOI: 10.3389/fnmol.2017.00199
Distinct Features of Doublecortin as a Marker of Neuronal Migration and Its Implications in Cancer Cell Mobility
Abstract
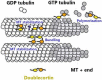
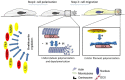
Neuronal migration is a critical process in the development of the nervous system. Defects in the migration of the neurons are associated with diseases like lissencephaly, subcortical band heterotopia (SBH), and pachygyria. Doublecortin (DCX) is an essential factor in neurogenesis and mutations in this protein impairs neuronal migration leading to several pathological conditions. Although, DCX is capable of modulating and stabilizing microtubules (MTs) to ensure effective migration, the mechanisms involved in executing these functions remain poorly understood. Meanwhile, there are existing gaps regarding the processes that underlie tumor initiation and progression into cancer as well as the ability to migrate and invade normal cells. Several studies suggest that DCX is involved in cancer metastasis. Unstable interactions between DCX and MTs destabilizes cytoskeletal organization leading to disorganized movements of cells, a process which may be implicated in the uncontrolled migration of cancer cells. However, the underlying mechanism is complex and require further clarification. Therefore, exploring the importance and features known up to date about this molecule will broaden our understanding and shed light on potential therapeutic approaches for the associated neurological diseases. This review summarizes current knowledge about DCX, its features, functions, and relationships with other proteins. We also present an overview of its role in cancer cells and highlight the importance of studying its gene mutations.
Keywords: CSC-cancer stem cells; DCX-doublecortin; GBM-glioblastoma multiforme; MAP-microtubule-associated protein; MT-microtubule; NSC-neural stem cells.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

