An Overview on Medicinal Chemistry of Synthetic and Natural Derivatives of Cannabidiol
- PMID: 28701957
- PMCID: PMC5487438
- DOI: 10.3389/fphar.2017.00422
An Overview on Medicinal Chemistry of Synthetic and Natural Derivatives of Cannabidiol
Abstract
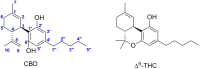
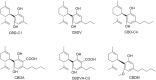
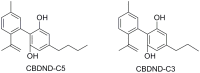
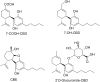
Cannabidiol (CBD) has been traditionally used in Cannabis-based preparation, however historically, it has received far less interest as a single drug than the other components of Cannabis. Currently, CBD generates considerable interest due to its beneficial neuroprotective, antiepileptic, anxiolytic, antipsychotic, and anti-inflammatory properties. Therefore, the CBD scaffold becomes of increasing interest for medicinal chemists. This review provides an overview of the chemical structure of natural and synthetic CBD derivatives including the molecular targets associated with these compounds. A clear identification of their biological targets has been shown to be still very challenging.
Keywords: cannabidiol; cannabidiol derivative; cannabinoid receptor; molecular target; therapeutic application.
Figures

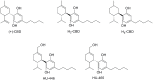

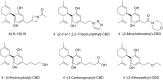
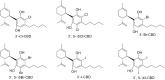
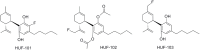
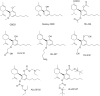
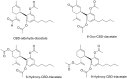
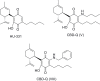
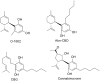
References
-
- Appendino G., Cabello de Alba M. L. B., Munoz Blanco E. (2015). Preparation of novel cannabidiol quinone derivatives as PPARγ agonists. International Patent No WO2015/158381 Washington, DC.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

