Role of Mitochondrial Reverse Electron Transport in ROS Signaling: Potential Roles in Health and Disease
- PMID: 28701960
- PMCID: PMC5486155
- DOI: 10.3389/fphys.2017.00428
Role of Mitochondrial Reverse Electron Transport in ROS Signaling: Potential Roles in Health and Disease
Abstract
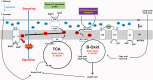
Reactive Oxygen Species (ROS) can cause oxidative damage and have been proposed to be the main cause of aging and age-related diseases including cancer, diabetes and Parkinson's disease. Accordingly, mitochondria from old individuals have higher levels of ROS. However, ROS also participate in cellular signaling, are instrumental for several physiological processes and boosting ROS levels in model organisms extends lifespan. The current consensus is that low levels of ROS are beneficial, facilitating adaptation to stress via signaling, whereas high levels of ROS are deleterious because they trigger oxidative stress. Based on this model the amount of ROS should determine the physiological effect. However, recent data suggests that the site at which ROS are generated is also instrumental in determining effects on cellular homeostasis. The best example of site-specific ROS signaling is reverse electron transport (RET). RET is produced when electrons from ubiquinol are transferred back to respiratory complex I, reducing NAD+ to NADH. This process generates a significant amount of ROS. RET has been shown to be instrumental for the activation of macrophages in response to bacterial infection, re-organization of the electron transport chain in response to changes in energy supply and adaptation of the carotid body to changes in oxygen levels. In Drosophila melanogaster, stimulating RET extends lifespan. Here, we review what is known about RET, as an example of site-specific ROS signaling, and its implications for the field of redox biology.
Keywords: ROS; complex I; mitochondria; redox signaling; reverse electron transport.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

