CRM1 Inhibitors for Antiviral Therapy
- PMID: 28702009
- PMCID: PMC5487384
- DOI: 10.3389/fmicb.2017.01171
CRM1 Inhibitors for Antiviral Therapy
Abstract
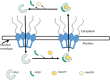
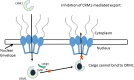
Infectious diseases are a major global concern and despite major advancements in medical research, still cause significant morbidity and mortality. Progress in antiviral therapy is particularly hindered by appearance of mutants capable of overcoming the effects of drugs targeting viral components. Alternatively, development of drugs targeting host proteins essential for completion of viral lifecycle holds potential as a viable strategy for antiviral therapy. Nucleocytoplasmic trafficking pathways in particular are involved in several pathological conditions including cancer and viral infections, where hijacking or alteration of function of key transporter proteins, such as Chromosome Region Maintenance1 (CRM1) is observed. Overexpression of CRM1-mediated nuclear export is evident in several solid and hematological malignancies. Interestingly, CRM1-mediated nuclear export of viral components is crucial in various stages of the viral lifecycle and assembly. This review summarizes the role of CRM1 in cancer and selected viruses. Leptomycin B (LMB) is the prototypical inhibitor of CRM1 potent against various cancer cell lines overexpressing CRM1 and in limiting viral infections at nanomolar concentrations in vitro. However, the irreversible shutdown of nuclear export results in high cytotoxicity and limited efficacy in vivo. This has prompted search for synthetic and natural CRM1 inhibitors that can potentially be developed as broadly active antivirals, some of which are summarized in this review.
Keywords: CRM1; CRM1 in cancer; CRM1 inhibitors; CRM1-mediated export of viral proteins; CRM1-mediated nuclear export.
Figures

Similar articles
-
Small Molecule Inhibitors of CRM1.Front Pharmacol. 2020 May 7;11:625. doi: 10.3389/fphar.2020.00625. eCollection 2020. Front Pharmacol. 2020. PMID: 32574233 Free PMC article. Review.
-
Nucleocytoplasmic Shuttling of Porcine Parvovirus NS1 Protein Mediated by the CRM1 Nuclear Export Pathway and the Importin α/β Nuclear Import Pathway.J Virol. 2022 Jan 12;96(1):e0148121. doi: 10.1128/JVI.01481-21. Epub 2021 Oct 13. J Virol. 2022. PMID: 34643426 Free PMC article.
-
Staufen-2 functions as a cofactor for enhanced Rev-mediated nucleocytoplasmic trafficking of HIV-1 genomic RNA via the CRM1 pathway.FEBS J. 2022 Nov;289(21):6731-6751. doi: 10.1111/febs.16546. Epub 2022 Jun 19. FEBS J. 2022. PMID: 35653259
-
Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1.Exp Cell Res. 1998 Aug 1;242(2):540-7. doi: 10.1006/excr.1998.4136. Exp Cell Res. 1998. PMID: 9683540
-
Inhibition of CRM1-dependent nuclear export sensitizes malignant cells to cytotoxic and targeted agents.Semin Cancer Biol. 2014 Aug;27:62-73. doi: 10.1016/j.semcancer.2014.03.001. Epub 2014 Mar 12. Semin Cancer Biol. 2014. PMID: 24631834 Free PMC article. Review.
Cited by
-
Identification of natural antiviral drug candidates against Tilapia Lake Virus: Computational drug design approaches.PLoS One. 2023 Nov 8;18(11):e0287944. doi: 10.1371/journal.pone.0287944. eCollection 2023. PLoS One. 2023. PMID: 37939069 Free PMC article.
-
Potential Anti-SARS-CoV-2 Therapeutics That Target the Post-Entry Stages of the Viral Life Cycle: A Comprehensive Review.Viruses. 2020 Sep 26;12(10):1092. doi: 10.3390/v12101092. Viruses. 2020. PMID: 32993173 Free PMC article. Review.
-
XPO1-dependent nuclear export as a target for cancer therapy.J Hematol Oncol. 2020 Jun 1;13(1):61. doi: 10.1186/s13045-020-00903-4. J Hematol Oncol. 2020. PMID: 32487143 Free PMC article. Review.
-
Effect of exportin 1/XPO1 nuclear export pathway inhibition on coronavirus replication.bioRxiv [Preprint]. 2024 Dec 11:2023.02.09.527884. doi: 10.1101/2023.02.09.527884. bioRxiv. 2024. Update in: Viruses. 2025 Feb 18;17(2):284. doi: 10.3390/v17020284. PMID: 36824761 Free PMC article. Updated. Preprint.
-
Viral Subversion of the Chromosome Region Maintenance 1 Export Pathway and Its Consequences for the Cell Host.Viruses. 2023 Nov 6;15(11):2218. doi: 10.3390/v15112218. Viruses. 2023. PMID: 38005895 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

