CaRDR1, an RNA-Dependent RNA Polymerase Plays a Positive Role in Pepper Resistance against TMV
- PMID: 28702034
- PMCID: PMC5487767
- DOI: 10.3389/fpls.2017.01068
CaRDR1, an RNA-Dependent RNA Polymerase Plays a Positive Role in Pepper Resistance against TMV
Abstract
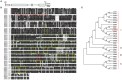
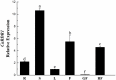
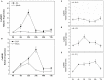
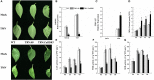
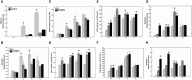
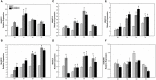
RNA silencing functions as a major natural antiviral defense mechanism in plants. RNA-dependent RNA polymerases (RDRs) that catalyze the synthesis of double-stranded RNAs, are considered as a fundamental element in RNA silencing pathways. In Arabidopsis thaliana, RDR1, 2 and 6 play important roles in anti-viral RNA silencing. Expression of RDR1 can be elevated following plant treatment with defense hormones and virus infection. RDR1 has been studied in several crop species, but not in pepper (Capsicum annuum L.). Here, a RDR1 gene was isolated from Capsicum annuum L., designated as CaRDR1. The full-length cDNA of CaRDR1 was 3,351 bp, encoding a 1,116-amino acid protein, which contains conserved regions, such as the most remarkable motif DLDGD. The transcripts of CaRDR1 could be induced by salicylic acid (SA), abscisic acid (ABA), H2O2, and tobacco mosaic virus (TMV). Silencing of CaRDR1 in pepper resulted in increased susceptibility to TMV as evident by severe symptom, increased of TMV-CP transcript, higher malondialdehyde (MDA) content and lower antioxidant enzymes activities compared with that of control plants. CaRDR1-overexpressing in Nicotiana benthamiana showed mild disease symptom and reduced TMV-CP transcripts than that of empty vector (EV) following TMV inoculation. The RNA silencing related genes, including NbAGO2, NbDCL2, NbDCL3, and NbDCL4 elevated expression in overexpressed plants. Alternative oxidase (AOX), the terminal oxidase of the cyanide (CN)-resistant alternative respiratory pathway, catalyze oxygen-dependent oxidation of ubiquinol in plants. It has an important function in plant defense against TMV. In addition, CaRDR1 overexpression promoted the expression of NbAOX1a and NbAOX1b. In conclusion, these results suggest that CaRDR1 plays a positive role in TMV resistance by regulating antioxidant enzymes activities and RNA silencing-related genes expression to suppress the replication and movement of TMV.
Keywords: CaRDR1; Nicotiana benthamiana; pepper; resistance; tobacco mosaic virus (TMV).
Figures

Similar articles
-
The relationship between the plant-encoded RNA-dependent RNA polymerase 1 and alternative oxidase in tomato basal defense against Tobacco mosaic virus.Planta. 2015 Mar;241(3):641-50. doi: 10.1007/s00425-014-2207-y. Epub 2014 Nov 19. Planta. 2015. PMID: 25408506
-
The role of hydrogen peroxide and nitric oxide in the induction of plant-encoded RNA-dependent RNA polymerase 1 in the basal defense against Tobacco mosaic virus.PLoS One. 2013 Sep 30;8(9):e76090. doi: 10.1371/journal.pone.0076090. eCollection 2013. PLoS One. 2013. PMID: 24098767 Free PMC article.
-
Salicylic acid treatment and expression of an RNA-dependent RNA polymerase 1 transgene inhibit lethal symptoms and meristem invasion during tobacco mosaic virus infection in Nicotiana benthamiana.BMC Plant Biol. 2016 Jan 13;16:15. doi: 10.1186/s12870-016-0705-8. BMC Plant Biol. 2016. PMID: 26757721 Free PMC article.
-
miR403a and SA Are Involved in NbAGO2 Mediated Antiviral Defenses Against TMV Infection in Nicotiana benthamiana.Genes (Basel). 2019 Jul 12;10(7):526. doi: 10.3390/genes10070526. Genes (Basel). 2019. PMID: 31336929 Free PMC article.
-
Replication of tobacco mosaic virus RNA.Philos Trans R Soc Lond B Biol Sci. 1999 Mar 29;354(1383):613-27. doi: 10.1098/rstb.1999.0413. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10212941 Free PMC article. Review.
Cited by
-
Molecular Regulation of Host Defense Responses Mediated by Biological Anti-TMV Agent Ningnanmycin.Viruses. 2019 Sep 3;11(9):815. doi: 10.3390/v11090815. Viruses. 2019. PMID: 31484426 Free PMC article.
-
RNA Interference: A Natural Immune System of Plants to Counteract Biotic Stressors.Cells. 2019 Jan 10;8(1):38. doi: 10.3390/cells8010038. Cells. 2019. PMID: 30634662 Free PMC article. Review.
-
Exploring the source of TYLCV resistance in Nicotiana benthamiana.Front Plant Sci. 2024 May 28;15:1404160. doi: 10.3389/fpls.2024.1404160. eCollection 2024. Front Plant Sci. 2024. PMID: 38863537 Free PMC article.
-
The Modulatory Role of sti-1 in Methylmercury-Induced Toxicity in Caenorhabditis elegans.Neurotox Res. 2022 Jun;40(3):837-846. doi: 10.1007/s12640-022-00515-5. Epub 2022 Apr 26. Neurotox Res. 2022. PMID: 35471723
-
Chimeric Tobamoviruses With Coat Protein Exchanges Modulate Symptom Expression and Defence Responses in Nicotiana tabacum.Front Microbiol. 2020 Nov 6;11:587005. doi: 10.3389/fmicb.2020.587005. eCollection 2020. Front Microbiol. 2020. PMID: 33240243 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

