Establishment of human hair follicle mesenchymal stem cells with overexpressed human hepatocyte growth factor
- PMID: 28702144
- PMCID: PMC5501929
- DOI: 10.22038/IJBMS.2017.8834
Establishment of human hair follicle mesenchymal stem cells with overexpressed human hepatocyte growth factor
Abstract
Objectives: Chronic liver disease has become a major health problem that causes serious damage to human health. Since the existing treatment effect was not ideal, we need to seek new treatment methods.
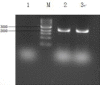
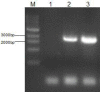
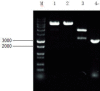
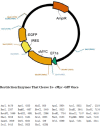
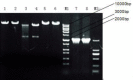
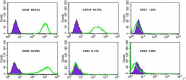
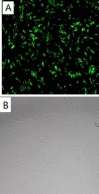
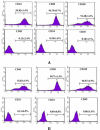
Materials and methods: We utilized the gene recombination technology to obtain the human hair mesenchymal stem cells which overexpression of human hepatocyte growth factor (hHGF). Furthermore, we verified the property of transfected cells through detecting surface marker by flow cytometry.
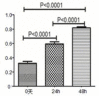
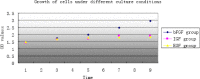
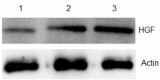
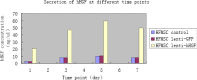
Results: We show here establishment of the hHGF-overexpressing lentivirus vector, and successfully transfection to human hair follicle mesenchymal stem cells. The verified experiments could demonstrate the human hair follicle mesenchymal stem cells which have been transfected still have the properties of stem cells.
Conclusion: We successfully constructed human hair follicle mesenchymal stem cells which overexpression hHGF, and maintain the same properties compared with pro-transfected cells.
Keywords: HGF; Hair follicle; Lentivirus; Liver diseases; Stem cells.
Figures







References
LinkOut - more resources
Full Text Sources
