Antioxidant System Response and cDNA-SCoT Marker Profiling in Phoenix dactylifera L. Plant under Salinity Stress
- PMID: 28702461
- PMCID: PMC5494121
- DOI: 10.1155/2017/1537538
Antioxidant System Response and cDNA-SCoT Marker Profiling in Phoenix dactylifera L. Plant under Salinity Stress
Abstract
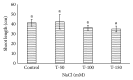
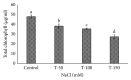
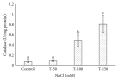
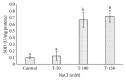
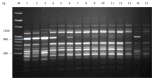
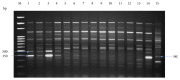
Many Phoenix dactylifera (date palm) cultivars are grown in the arid and semiarid regions of the world, including Saudi Arabia. P. dactylifera is highly tolerant to salinity stress. To investigate the response of Khalas cultivar of P. dactylifera, two-month-old plants were treated with sodium chloride (50, 100, and 150 mM NaCl) for three months. Our result showed that proline content was higher in all treated plants compared to control plants. Thiobarbituric acid reactive substances (TBARS) were increased at 100 and 150 mM NaCl treatments; however, the result was found nonsignificant between control and plants treated at 50 mM NaCl. Similarly, enzyme activities of catalase (CAT) and superoxide dismutase (SOD) were 0.805 and 0.722 U/mg protein/min, respectively, and were greater at 100 and 150 mM NaCl treatments compared to the control plants. Total chlorophyll content and fresh weight of shoots and roots decreased substantially with the increase of salinity. A cDNA start codon-targeted (cDNA-SCoT) marker showed a variation in different gene expressions profiling between treated and untreated plants under various NaCl concentrations.
Figures







References
-
- Tanji K. K. Nature and extent of agricultural salinity. In: Tangi K. K., editor. Agricultural Salinity Assessment and Management. New York: American Society of Civil Engineers; 1990. pp. 1–17.
-
- Szabolcs I. Soil salinization. In: Pessarakli M., editor. Handbook of Plant Crop Stress. New York: Marcel Dekker; 1994. pp. 3–11.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

