Implications of peptide assemblies in amyloid diseases
- PMID: 28702523
- PMCID: PMC5902192
- DOI: 10.1039/c7cs00372b
Implications of peptide assemblies in amyloid diseases
Abstract
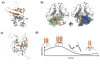
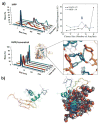
Neurodegenerative disorders and type 2 diabetes are global epidemics compromising the quality of life of millions worldwide, with profound social and economic implications. Despite the significant differences in pathology - much of which are poorly understood - these diseases are commonly characterized by the presence of cross-β amyloid fibrils as well as the loss of neuronal or pancreatic β-cells. In this review, we document research progress on the molecular and mesoscopic self-assembly of amyloid-beta, alpha synuclein, human islet amyloid polypeptide and prions, the peptides and proteins associated with Alzheimer's, Parkinson's, type 2 diabetes and prion diseases. In addition, we discuss the toxicities of these amyloid proteins based on their self-assembly as well as their interactions with membranes, metal ions, small molecules and engineered nanoparticles. Through this presentation we show the remarkable similarities and differences in the structural transitions of the amyloid proteins through primary and secondary nucleation, the common evolution from disordered monomers to alpha-helices and then to β-sheets when the proteins encounter the cell membrane, and, the consensus (with a few exceptions) that off-pathway oligomers, rather than amyloid fibrils, are the toxic species regardless of the pathogenic protein sequence or physicochemical properties. In addition, we highlight the crucial role of molecular self-assembly in eliciting the biological and pathological consequences of the amyloid proteins within the context of their cellular environments and their spreading between cells and organs. Exploiting such structure-function-toxicity relationship may prove pivotal for the detection and mitigation of amyloid diseases.
Figures

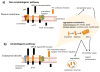














Similar articles
-
Elucidating the Structures of Amyloid Oligomers with Macrocyclic β-Hairpin Peptides: Insights into Alzheimer's Disease and Other Amyloid Diseases.Acc Chem Res. 2018 Mar 20;51(3):706-718. doi: 10.1021/acs.accounts.7b00554. Epub 2018 Mar 6. Acc Chem Res. 2018. PMID: 29508987 Free PMC article. Review.
-
Mixed pathologies in pancreatic β cells from subjects with neurodegenerative diseases and their interaction with prion protein.Acta Neuropathol Commun. 2021 Apr 8;9(1):64. doi: 10.1186/s40478-021-01171-0. Acta Neuropathol Commun. 2021. PMID: 33832546 Free PMC article.
-
Phthalocyanines as Molecular Scaffolds to Block Disease-Associated Protein Aggregation.Acc Chem Res. 2016 May 17;49(5):801-8. doi: 10.1021/acs.accounts.5b00507. Epub 2016 May 2. Acc Chem Res. 2016. PMID: 27136297 Review.
-
Amyloid Oligomers: A Joint Experimental/Computational Perspective on Alzheimer's Disease, Parkinson's Disease, Type II Diabetes, and Amyotrophic Lateral Sclerosis.Chem Rev. 2021 Feb 24;121(4):2545-2647. doi: 10.1021/acs.chemrev.0c01122. Epub 2021 Feb 5. Chem Rev. 2021. PMID: 33543942 Free PMC article. Review.
-
A unifying framework for amyloid-mediated membrane damage: The lipid-chaperone hypothesis.Biochim Biophys Acta Proteins Proteom. 2022 Apr 1;1870(4):140767. doi: 10.1016/j.bbapap.2022.140767. Epub 2022 Feb 8. Biochim Biophys Acta Proteins Proteom. 2022. PMID: 35144022 Review.
Cited by
-
Mitigation of Amyloidosis with Nanomaterials.Adv Mater. 2020 May;32(18):e1901690. doi: 10.1002/adma.201901690. Epub 2019 Jun 11. Adv Mater. 2020. PMID: 31183916 Free PMC article. Review.
-
Photoluminescent nanocluster-based probes for bioimaging applications.Photochem Photobiol Sci. 2022 May;21(5):787-801. doi: 10.1007/s43630-021-00153-4. Epub 2022 Jan 15. Photochem Photobiol Sci. 2022. PMID: 35032005 Review.
-
The membrane axis of Alzheimer's nanomedicine.Adv Nanobiomed Res. 2021 Jan;1(1):2000040. doi: 10.1002/anbr.202000040. Epub 2020 Nov 26. Adv Nanobiomed Res. 2021. PMID: 33748816 Free PMC article.
-
Phosphorylation of covalent organic framework nanospheres for inhibition of amyloid-β peptide fibrillation.Chem Sci. 2022 Apr 22;13(20):5902-5912. doi: 10.1039/d2sc00253a. eCollection 2022 May 25. Chem Sci. 2022. PMID: 35685783 Free PMC article.
-
Direct Observation of Seeded Conformational Conversion of hIAPP In Silico Reveals the Mechanisms for Morphological Dependence and Asymmetry of Fibril Growth.J Chem Inf Model. 2023 Sep 25;63(18):5863-5873. doi: 10.1021/acs.jcim.3c00898. Epub 2023 Aug 31. J Chem Inf Model. 2023. PMID: 37651616 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

