Flat clathrin lattices are dynamic actin-controlled hubs for clathrin-mediated endocytosis and signalling of specific receptors
- PMID: 28703125
- PMCID: PMC5511353
- DOI: 10.1038/ncomms16068
Flat clathrin lattices are dynamic actin-controlled hubs for clathrin-mediated endocytosis and signalling of specific receptors
Abstract
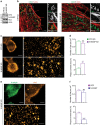
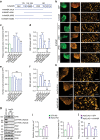
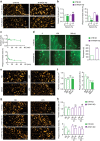
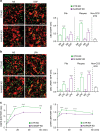
Clathrin lattices at the plasma membrane coat both invaginated and flat regions forming clathrin-coated pits and clathrin plaques, respectively. The function and regulation of clathrin-coated pits in endocytosis are well understood but clathrin plaques remain enigmatic nanodomains. Here we use super-resolution microscopy, molecular genetics and cell biology to show that clathrin plaques contain the machinery for clathrin-mediated endocytosis and cell adhesion, and associate with both clathrin-coated pits and filamentous actin. We also find that actin polymerization promoted by N-WASP through the Arp2/3 complex is crucial for the regulation of plaques but not pits. Clathrin plaques oppose cell migration and undergo actin- and N-WASP-dependent disassembly upon activation of LPA receptor 1, but not EGF receptor. Most importantly, plaque disassembly correlates with the endocytosis of LPA receptor 1 and down-modulation of AKT activity. Thus, clathrin plaques serve as dynamic actin-controlled hubs for clathrin-mediated endocytosis and signalling that exhibit receptor specificity.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
N-WASP deficiency impairs EGF internalization and actin assembly at clathrin-coated pits.J Cell Sci. 2005 Jul 15;118(Pt 14):3103-15. doi: 10.1242/jcs.02444. Epub 2005 Jun 28. J Cell Sci. 2005. PMID: 15985465
-
A Flat BAR Protein Promotes Actin Polymerization at the Base of Clathrin-Coated Pits.Cell. 2018 Jul 12;174(2):325-337.e14. doi: 10.1016/j.cell.2018.05.020. Epub 2018 Jun 7. Cell. 2018. PMID: 29887380 Free PMC article.
-
Interplay between N-WASP and CK2 optimizes clathrin-mediated endocytosis of EGFR.J Cell Sci. 2011 Jun 15;124(Pt 12):2001-12. doi: 10.1242/jcs.081182. Epub 2011 May 24. J Cell Sci. 2011. PMID: 21610097
-
Myosin VI, a new force in clathrin mediated endocytosis.FEBS Lett. 2001 Nov 23;508(3):295-9. doi: 10.1016/s0014-5793(01)03065-4. FEBS Lett. 2001. PMID: 11728438 Review.
-
Endocytosis: clathrin-mediated membrane budding.Curr Opin Cell Biol. 2007 Aug;19(4):417-25. doi: 10.1016/j.ceb.2007.05.003. Epub 2007 Jul 13. Curr Opin Cell Biol. 2007. PMID: 17631994 Review.
Cited by
-
The live cell DNA stain SiR-Hoechst induces DNA damage responses and impairs cell cycle progression.Sci Rep. 2018 May 21;8(1):7898. doi: 10.1038/s41598-018-26307-6. Sci Rep. 2018. PMID: 29785044 Free PMC article.
-
Clathrin-containing adhesion complexes.J Cell Biol. 2019 Jul 1;218(7):2086-2095. doi: 10.1083/jcb.201811160. Epub 2019 Jun 17. J Cell Biol. 2019. PMID: 31208994 Free PMC article. Review.
-
Dual clathrin and integrin signaling systems regulate growth factor receptor activation.Nat Commun. 2022 Feb 16;13(1):905. doi: 10.1038/s41467-022-28373-x. Nat Commun. 2022. PMID: 35173166 Free PMC article.
-
Reticular adhesions are assembled at flat clathrin lattices and opposed by active integrin α5β1.J Cell Biol. 2023 Aug 7;222(8):e202303107. doi: 10.1083/jcb.202303107. Epub 2023 May 26. J Cell Biol. 2023. PMID: 37233325 Free PMC article.
-
Substrate Stiffness-Driven Membrane Tension Modulates Vesicular Trafficking via Caveolin-1.ACS Nano. 2022 Mar 22;16(3):4322-4337. doi: 10.1021/acsnano.1c10534. Epub 2022 Mar 7. ACS Nano. 2022. PMID: 35255206 Free PMC article.
References
-
- McMahon H. T. & Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. rev. Mol. cell biol. 12, 517–533 (2011). - PubMed
-
- Ehrlich M. et al.. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118, 591–605 (2004). - PubMed
-
- Laroche G., Rochdi M. D., Laporte S. A. & Parent J. L. Involvement of actin in agonist-induced endocytosis of the G protein-coupled receptor for thromboxane A2: overcoming of actin disruption by arrestin-3 but not arrestin-2. J. biol. chem. 280, 23215–23224 (2005). - PubMed
-
- Wolfe B. L. & Trejo J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic 8, 462–470 (2007). - PubMed
-
- Fujimoto L. M., Roth R., Heuser J. E. & Schmid S. L. Actin assembly plays a variable, but not obligatory role in receptor-mediated endocytosis in mammalian cells. Traffic 1, 161–171 (2000). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

