Quantitative real-time imaging of glutathione
- PMID: 28703127
- PMCID: PMC5511354
- DOI: 10.1038/ncomms16087
Quantitative real-time imaging of glutathione
Erratum in
-
Corrigendum: Quantitative real-time imaging of glutathione.Nat Commun. 2017 Oct 3;8:16163. doi: 10.1038/ncomms16163. Nat Commun. 2017. PMID: 28972204 Free PMC article.
Abstract
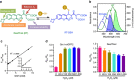
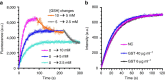
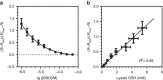
Glutathione plays many important roles in biological processes; however, the dynamic changes of glutathione concentrations in living cells remain largely unknown. Here, we report a reversible reaction-based fluorescent probe-designated as RealThiol (RT)-that can quantitatively monitor the real-time glutathione dynamics in living cells. Using RT, we observe enhanced antioxidant capability of activated neurons and dynamic glutathione changes during ferroptosis. RT is thus a versatile tool that can be used for both confocal microscopy and flow cytometry based high-throughput quantification of glutathione levels in single cells. We envision that this new glutathione probe will enable opportunities to study glutathione dynamics and transportation and expand our understanding of the physiological and pathological roles of glutathione in living cells.
Conflict of interest statement
X.J., J.C. and J.W. are co-inventors of a patent application related to this work. The remaining authors declare no competing financial interests.
Figures





Comment in
-
Pitfalls in the quantitative imaging of glutathione in living cells.Nat Commun. 2018 Apr 23;9(1):1588. doi: 10.1038/s41467-018-04035-9. Nat Commun. 2018. PMID: 29686354 Free PMC article. No abstract available.
References
-
- Ghezzi P. Protein glutathionylation in health and disease. Biochim. Biophys. Acta 1830, 3165–3172 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS100893/NS/NINDS NIH HHS/United States
- R21 CA213535/CA/NCI NIH HHS/United States
- R01 GM120033/GM/NIGMS NIH HHS/United States
- U54 HD083092/HD/NICHD NIH HHS/United States
- P30 HD024064/HD/NICHD NIH HHS/United States
- P30 CA125123/CA/NCI NIH HHS/United States
- P30 AI036211/AI/NIAID NIH HHS/United States
- R01 AG045183/AG/NIA NIH HHS/United States
- S10 RR024574/RR/NCRR NIH HHS/United States
- DP1 DK113644/DK/NIDDK NIH HHS/United States
- R21 EB022302/EB/NIBIB NIH HHS/United States
- R01 GM115622/GM/NIGMS NIH HHS/United States
- R01 AT009050/AT/NCCIH NIH HHS/United States
- R01 CA207701/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

