The Effect of Renal Impairment on the Pharmacokinetics and Pharmacodynamics of Ertugliflozin in Subjects With Type 2 Diabetes Mellitus
- PMID: 28703316
- PMCID: PMC5655776
- DOI: 10.1002/jcph.955
The Effect of Renal Impairment on the Pharmacokinetics and Pharmacodynamics of Ertugliflozin in Subjects With Type 2 Diabetes Mellitus
Abstract
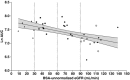
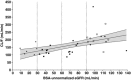
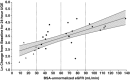
Ertugliflozin is a highly selective and potent inhibitor of the sodium-glucose cotransporter 2 in development for the treatment of type 2 diabetes mellitus. The glycemic efficacy of sodium-glucose cotransporter 2 inhibitors such as ertugliflozin depends on glucose filtration through the kidney. This phase 1, open-label study evaluated the effect of renal impairment on the pharmacokinetics, pharmacodynamics, and tolerability of ertugliflozin (15 mg) in type 2 diabetes mellitus and healthy subjects with normal renal function (estimated glomerular filtration rate not normalized for body surface area ≥90 mL/min) and type 2 diabetes mellitus subjects with mild (60-89 mL/min), moderate (30-59 mL/min), or severe (<30 mL/min) renal impairment (n = 36). Blood and urine samples were collected predose and over 96 hours postdose for pharmacokinetic evaluation and measurement of urinary glucose excretion over 24 hours. Log-linear regression analyses indicated predicted mean area under the concentration-time curve values for mild, moderate, and severe renal function groups that were ≤70% higher relative to subjects with normal renal function. Generally consistent results were obtained with categorical analysis based on analysis of variance. The increase in ertugliflozin exposure in subjects with renal impairment is not expected to be clinically meaningful. Regression analysis of change from baseline in urinary glucose excretion over 24 hours vs estimated glomerular filtration rate showed a decrease in urinary glucose excretion with declining renal function. A single 15-mg dose of ertugliflozin was well tolerated in all groups.
Keywords: ertugliflozin; renal impairment; sodium-glucose cotransporter 2; type 2 diabetes mellitus.
© 2017 The Authors. The Journal of Clinical Pharmacology Published by Wiley Periodicals, Inc. on behalf of American College of Clinical Pharmacology.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

