Cudraflavone C Induces Apoptosis of A375.S2 Melanoma Cells through Mitochondrial ROS Production and MAPK Activation
- PMID: 28703746
- PMCID: PMC5535998
- DOI: 10.3390/ijms18071508
Cudraflavone C Induces Apoptosis of A375.S2 Melanoma Cells through Mitochondrial ROS Production and MAPK Activation
Abstract
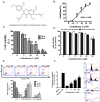
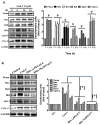
Melanoma is the most malignant form of skin cancer and is associated with a very poor prognosis. The aim of this study was to evaluate the apoptotic effects of cudraflavone C on A375.S2 melanoma cells and to determine the underlying mechanisms involved in apoptosis. Cell viability was determined using the MTT and real-time cytotoxicity assays. Flow cytometric evaluation of apoptosis was performed after staining the cells with Annexin V-FITC and propidium iodide. The mitochondrial membrane potential was evaluated using the JC-1 assay. Cellular ROS production was measured using the CellROX assay, while mitochondrial ROS production was evaluated using the MitoSOX assay. It was observed that cudraflavone C inhibited growth in A375.S2 melanoma cells, and promoted apoptosis via the mitochondrial pathway mediated by increased mitochondrial ROS production. In addition, cudraflavone C induced phosphorylation of MAPKs (p38, ERK, and JNK) and up-regulated the expression of apoptotic proteins (Puma, Bax, Bad, Bid, Apaf-1, cytochrome C, caspase-9, and caspase-3/7) in A375.S2 cells. Pretreatment of A375.S2 cells with MitoTEMPOL (a mitochondria-targeted antioxidant) attenuated the phosphorylation of MAPKs, expression of apoptotic proteins, and the overall progression of apoptosis. In summary, cudraflavone C induced apoptosis in A375.S2 melanoma cells by increasing mitochondrial ROS production; thus, activating p38, ERK, and JNK; and increasing the expression of apoptotic proteins. Therefore, cudraflavone C may be regarded as a potential form of treatment for malignant melanoma.
Keywords: MAPKs; apoptosis; cudraflavone C; melanoma cells; mitochondria; pro-oxidation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Benzyl isothiocyanate (BITC) induces G2/M phase arrest and apoptosis in human melanoma A375.S2 cells through reactive oxygen species (ROS) and both mitochondria-dependent and death receptor-mediated multiple signaling pathways.J Agric Food Chem. 2012 Jan 18;60(2):665-75. doi: 10.1021/jf204193v. Epub 2012 Jan 6. J Agric Food Chem. 2012. PMID: 22148415
-
Phenethyl isothiocyanate triggers apoptosis in human malignant melanoma A375.S2 cells through reactive oxygen species and the mitochondria-dependent pathways.Hum Exp Toxicol. 2014 Mar;33(3):270-83. doi: 10.1177/0960327113491508. Epub 2013 Jun 11. Hum Exp Toxicol. 2014. PMID: 23760257
-
7-O-Geranylquercetin induces apoptosis in gastric cancer cells via ROS-MAPK mediated mitochondrial signaling pathway activation.Biomed Pharmacother. 2017 Mar;87:527-538. doi: 10.1016/j.biopha.2016.12.095. Epub 2017 Jan 8. Biomed Pharmacother. 2017. PMID: 28076833
-
Molecular pathways supporting the proliferation staging of malignant melanoma (review).Int J Mol Med. 2009 Sep;24(3):295-301. doi: 10.3892/ijmm_00000232. Int J Mol Med. 2009. PMID: 19639220 Review.
-
Key regulators of apoptosis execution as biomarker candidates in melanoma.Mol Cell Oncol. 2014 Dec 23;1(3):e964037. doi: 10.4161/23723548.2014.964037. eCollection 2014 Jul-Sep. Mol Cell Oncol. 2014. PMID: 27308353 Free PMC article. Review.
Cited by
-
Emerging Perspective: Role of Increased ROS and Redox Imbalance in Skin Carcinogenesis.Oxid Med Cell Longev. 2019 Sep 16;2019:8127362. doi: 10.1155/2019/8127362. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31636809 Free PMC article. Review.
-
Disruption of Redox Homeostasis by Alterations in Nitric Oxide Synthase Activity and Tetrahydrobiopterin along with Melanoma Progression.Int J Mol Sci. 2022 May 26;23(11):5979. doi: 10.3390/ijms23115979. Int J Mol Sci. 2022. PMID: 35682659 Free PMC article.
-
Greensporone A, a Fungal Secondary Metabolite Suppressed Constitutively Activated AKT via ROS Generation and Induced Apoptosis in Leukemic Cell Lines.Biomolecules. 2019 Mar 29;9(4):126. doi: 10.3390/biom9040126. Biomolecules. 2019. PMID: 30934922 Free PMC article.
-
Mechanism of Lakoochin A Inducing Apoptosis of A375.S2 Melanoma Cells through Mitochondrial ROS and MAPKs Pathway.Int J Mol Sci. 2018 Sep 6;19(9):2649. doi: 10.3390/ijms19092649. Int J Mol Sci. 2018. PMID: 30200660 Free PMC article.
-
Greensporone C, a Freshwater Fungal Secondary Metabolite Induces Mitochondrial-Mediated Apoptotic Cell Death in Leukemic Cell Lines.Front Pharmacol. 2018 Jul 16;9:720. doi: 10.3389/fphar.2018.00720. eCollection 2018. Front Pharmacol. 2018. PMID: 30061828 Free PMC article.
References
-
- Sritularak B., Tantrakarnsakul K., Lipipun V., Likhitwitayawuid K. Flavonoids with anti-HSV activity from the root bark of Artocarpus lakoocha. Nat. Prod. Commun. 2013;8:1079–1080. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

