A Chemist's Perspective on the Role of Phosphorus at the Origins of Life
- PMID: 28703763
- PMCID: PMC5617956
- DOI: 10.3390/life7030031
A Chemist's Perspective on the Role of Phosphorus at the Origins of Life
Abstract
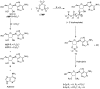
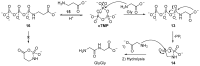
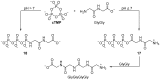
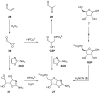
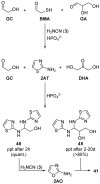
The central role that phosphates play in biological systems, suggests they also played an important role in the emergence of life on Earth. In recent years, numerous important advances have been made towards understanding the influence that phosphates may have had on prebiotic chemistry, and here, we highlight two important aspects of prebiotic phosphate chemistry. Firstly, we discuss prebiotic phosphorylation reactions; we specifically contrast aqueous electrophilic phosphorylation, and aqueous nucleophilic phosphorylation strategies, with dry-state phosphorylations that are mediated by dissociative phosphoryl-transfer. Secondly, we discuss the non-structural roles that phosphates can play in prebiotic chemistry. Here, we focus on the mechanisms by which phosphate has guided prebiotic reactivity through catalysis or buffering effects, to facilitating selective transformations in neutral water. Several prebiotic routes towards the synthesis of nucleotides, amino acids, and core metabolites, that have been facilitated or controlled by phosphate acting as a general acid-base catalyst, pH buffer, or a chemical buffer, are outlined. These facile and subtle mechanisms for incorporation and exploitation of phosphates to orchestrate selective, robust prebiotic chemistry, coupled with the central and universally conserved roles of phosphates in biochemistry, provide an increasingly clear message that understanding phosphate chemistry will be a key element in elucidating the origins of life on Earth.
Keywords: amino acids; general acid-base catalyst.; nucleotides; phosphate; phosphorylation; prebiotic chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

