Understanding the Inguinal Sinus in Sheep (Ovis aries)-Morphology, Secretion, and Expression of Progesterone, Estrogens, and Prolactin Receptors
- PMID: 28703772
- PMCID: PMC5536006
- DOI: 10.3390/ijms18071516
Understanding the Inguinal Sinus in Sheep (Ovis aries)-Morphology, Secretion, and Expression of Progesterone, Estrogens, and Prolactin Receptors
Abstract
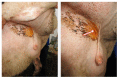
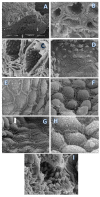
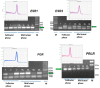
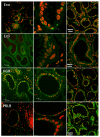
Post-parturient behavior of mammalian females is essential for early parent-offspring contact. After delivery, lambs need to ingest colostrum for obtaining the related immunological protection, and early interactions between the mother and the lamb are crucial. Despite visual and auditory cues, olfactory cues are decisive in lamb orientation to the mammary gland. In sheep, the inguinal sinus is located bilaterally near the mammary gland as a skin pouch (IGS) that presents a gland that secretes a strong-smelling wax. Sheep IGS gland functions have many aspects under evaluation. The objective of the present study was to evaluate sheep IGS gland functional aspects and mRNA transcription and the protein expression of several hormone receptors, such as progesterone receptor (PGR), estrogen receptor 1 (ESR1), and 2 (ESR2) and prolactin receptor (PRLR) present. In addition, another aim was to achieve information about IGS ultrastructure and chemical compounds produced in this gland. All hormone receptors evaluated show expression in IGS during the estrous cycle (follicular/luteal phases), pregnancy, and the post-partum period. IGS secretion is rich in triterpenoids that totally differ from the surrounding skin. They might be essential substances for the development of an olfactory preference of newborns to their mothers.
Keywords: ESR1; ESR2; PGR; PRLR; chemical compounds; inguinal sinus; morphology; transcription; triterpenoids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Endocrine control of canine mammary neoplasms: serum reproductive hormone levels and tissue expression of steroid hormone, prolactin and growth hormone receptors.BMC Vet Res. 2015 Sep 15;11:235. doi: 10.1186/s12917-015-0546-y. BMC Vet Res. 2015. PMID: 26370564 Free PMC article.
-
Transcriptional and spatiotemporal regulation of prolactin receptor mRNA and cooperativity with progesterone receptor function during ductal branch growth in the mammary gland.Dev Dyn. 2001 Oct;222(2):192-205. doi: 10.1002/dvdy.1179. Dev Dyn. 2001. PMID: 11668597
-
Bovine luteal prolactin receptor expression: potential involvement in regulation of progesterone during the estrous cycle and pregnancy.J Anim Sci. 2011 May;89(5):1338-46. doi: 10.2527/jas.2010-3559. J Anim Sci. 2011. PMID: 21521814
-
Regulation of gene expression in the bovine mammary gland by ovarian steroids.J Dairy Sci. 2007 Jun;90 Suppl 1:E55-65. doi: 10.3168/jds.2006-466. J Dairy Sci. 2007. PMID: 17517752 Review.
-
Endocrine hormones and local signals during the development of the mouse mammary gland.Wiley Interdiscip Rev Dev Biol. 2015 May-Jun;4(3):181-95. doi: 10.1002/wdev.172. Epub 2015 Jan 23. Wiley Interdiscip Rev Dev Biol. 2015. PMID: 25645332 Review.
Cited by
-
Molecular Pathways of Estrogen Receptor Action.Int J Mol Sci. 2018 Aug 31;19(9):2591. doi: 10.3390/ijms19092591. Int J Mol Sci. 2018. PMID: 30200344 Free PMC article. No abstract available.
References
-
- Brockman J.H., Snowdon C., Roper T., Naguib M., Wynne-Eduards K. Advances in the Study of Behavior. Volume 36. Elsevier Inc.; San Diego, CA, USA: 2009.
-
- Fuchs S. Optimality of parental investment: The influence of nursing on reproductive success of mother and female young house mice. Behav. Ecol. Sociobiol. 1982;10:39–51. doi: 10.1007/BF00296394. - DOI
-
- Schaal B. In: Pheromones for Newborns. Mucignat-Caretta C., Raton B., editors. Taylor & Francis; Abingdon, UK: 2014. Chapter 17. ISBN-13: 978-1-4665-5341. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

