EFFICACY AND SAFETY OF RANIBIZUMAB FOR THE TREATMENT OF CHOROIDAL NEOVASCULARIZATION DUE TO UNCOMMON CAUSE: Twelve-Month Results of the MINERVA Study
- PMID: 28704254
- PMCID: PMC6086222
- DOI: 10.1097/IAE.0000000000001744
EFFICACY AND SAFETY OF RANIBIZUMAB FOR THE TREATMENT OF CHOROIDAL NEOVASCULARIZATION DUE TO UNCOMMON CAUSE: Twelve-Month Results of the MINERVA Study
Abstract
Purpose: To evaluate the efficacy and safety of ranibizumab 0.5 mg in adult patients with choroidal neovascularization because of an uncommon cause enrolled in the 12-month MINERVA study.
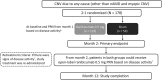
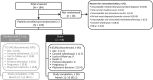
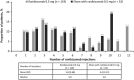
Methods: In this Phase III, double-masked study, adult (≥18 years) patients (N = 178) were randomized 2:1 to receive either ranibizumab (n = 119) or sham (n = 59) at baseline and, if needed, at Month 1 and open-label individualized ranibizumab from Month 2. Best-corrected visual acuity change from baseline to Month 2 (primary endpoint) and Month 12, treatment exposure, and safety over 12 months were reported. Subgroup analysis was conducted on five predefined choroidal neovascularization etiologies (angioid streak, postinflammatory, central serous chorioretinopathy, idiopathic, and miscellaneous).
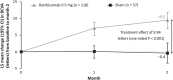
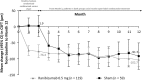
Results: Ranibizumab showed superior efficacy versus sham from baseline to Month 2 (adjusted least-squares mean best-corrected visual acuity: +9.5 vs. -0.4 letters; P < 0.001). At Month 12, the mean best-corrected visual acuity change was +11.0 letters (ranibizumab) and +9.3 letters (sham). Across the 5 subgroups, the treatment effect ranged from +5.0 to +14.6 letters. The mean number of ranibizumab injections was 5.8 (ranibizumab arm) with no new ocular or nonocular adverse events.
Conclusion: Ranibizumab 0.5 mg resulted in clinically significant treatment effect versus sham at Month 2. Overall, ranibizumab was effective in treating choroidal neovascularization of various etiologies with no new safety findings.
Figures



References
-
- Grossniklaus HE, Gass JD. Clincopathologic correlations of surgically excised type 1 and type 2 submacular choroidal neovsacular membranes. Am J Ophthalmol 1998;126:59–69. - PubMed
-
- Carneiro AM, Silva RM, Veludo MJ, et al. Ranibizumab treatment for choroidal neovascularization from causes other than age-related macular degeneration and pathological myopia. Ophthalmologica 2011;225:81–88. - PubMed
-
- Sivaprasad S, Moore AT. Choroidal neovascularisation in children. Br J Ophthalmol 2008;92:451–454. - PubMed
-
- Cohen SY, Laroche A, Leguen Y, et al. Etiology of choroidal neovascularization in young patients. Ophthalmology 1996;103:1241–1244. - PubMed
-
- Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol 2004;137:486–495. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

