Sofosbuvir based treatment of chronic hepatitis C genotype 3 infections-A Scandinavian real-life study
- PMID: 28704381
- PMCID: PMC5509146
- DOI: 10.1371/journal.pone.0179764
Sofosbuvir based treatment of chronic hepatitis C genotype 3 infections-A Scandinavian real-life study
Abstract
Background and aims: Chronic hepatitis C virus (HCV) genotype 3 infection with advanced liver disease has emerged as the most challenging to treat. We retrospectively assessed the treatment outcome of sofosbuvir (SOF) based regimes for treatment of HCV genotype 3 infections in a real life setting in Scandinavia.
Methods: Consecutive patients with chronic HCV genotype 3 infection were enrolled at 16 treatment centers in Denmark, Sweden, Norway and Finland. Patients who had received a SOF containing regimen were included. The fibrosis stage was evaluated by liver biopsy or transient liver elastography. The following treatments were given according availability and local guidelines: 1) SOF + ribavirin (RBV) for 24 weeks, 2) SOF + daclatasvir (DCV) +/-RBV for 12-24 weeks, 3) SOF + pegylated interferon alpha (peg-IFN-α) + RBV for 12 weeks or 4) SOF/ledipasvir (LDV) + RBV for 12-16 weeks. The primary endpoint was sustained virological response (SVR) assessed at week 12 (SVR12) after end of treatment.
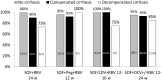
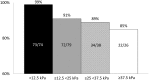
Results: We included 316 patients with a mean age of 55 years (range 24-79), 70% men, 49% treatment experienced, 58% with compensated cirrhosis and 12% with decompensated cirrhosis.In the modified intention to treat (mITT) population SVR12 was achieved in 284/311 (91%) patients. Among 26 treatment failures, five had non-response, 3 breakthrough and 18 relapse. Five patients were not included in the mITT population. Three patients died from reasons unrelated to treatment and two were lost to follow-up. The SVR12 rate was similar for all treatment regimens, but lower in men (p = 0.042), and in patients with decompensated liver disease (p = 0.004).
Conclusion: We found that sofosbuvir based treatment in a real-life setting could offer SVR rates exceeding 90% in patients with HCV genotype 3 infection and advanced liver disease.
Conflict of interest statement
Figures
References
-
- Cornberg M, Razavi HA, Alberti A, Bernasconi E, Buti M, Cooper C, et al. A systematic review of hepatitis C virus epidemiology in Europe, Canada and Israel. Liver Int. 2011;31 Suppl 2:30–60. Epub 2011/06/18. doi: 10.1111/j.1478-3231.2011.02539.x . - DOI - PubMed
-
- Bruggmann P, Berg T, Ovrehus AL, Moreno C, Brandao Mello CE, Roudot-Thoraval F, et al. Historical epidemiology of hepatitis C virus (HCV) in selected countries. J Viral Hepat. 2014;21 Suppl 1:5–33. doi: 10.1111/jvh.12247 . - DOI - PubMed
-
- Kanwal F, Kramer JR, Ilyas J, Duan Z, El-Serag HB. HCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of U.S. Veterans with HCV. Hepatology. 2014;60(1):98–105. doi: 10.1002/hep.27095 ; - DOI - PMC - PubMed
-
- Poordad F, McCone J Jr., Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 364(13):1195–206. Epub 2011/04/01. doi: 10.1056/NEJMoa1010494 . - DOI - PMC - PubMed
-
- Poordad F, McCone J Jr., Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1195–206. Epub 2011/04/01. doi: 10.1056/NEJMoa1010494 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

