Immunoprotective properties of recombinant LigA and LigB in a hamster model of acute leptospirosis
- PMID: 28704385
- PMCID: PMC5509140
- DOI: 10.1371/journal.pone.0180004
Immunoprotective properties of recombinant LigA and LigB in a hamster model of acute leptospirosis
Abstract
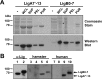
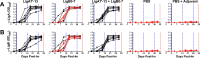
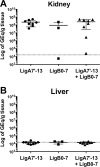
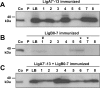
Leptospirosis is the most widespread zoonosis and is considered a major public health problem worldwide. Currently, there is no widely available vaccine against leptospirosis for use in humans. A purified, recombinant subunit vaccine that includes the last six immunoglobulin-like (Ig-like) domains of the leptospiral protein LigA (LigA7'-13) protects against lethal infection but not renal colonization after challenge by Leptospira interrogans. In this study, we examined whether the addition of the first seven Ig-like domains of LigB (LigB0-7) to LigA7'-13, can enhance immune protection and confer sterilizing immunity in the Golden Syrian hamster model of acute leptospirosis. Hamsters were subcutaneously immunized with soluble, recombinant LigA7'-13, LigB0-7, or a combination of LigA7'-13 and LigB0-7 in Freund's adjuvant. Immunization with Lig proteins generated a strong humoral immune response with high titers of IgG that recognized homologous protein, and cross-reacted with the heterologous protein as assessed by ELISA. LigA7'-13 alone, or in combination with LigB0-7, protected all hamsters from intraperitoneal challenge with a lethal dose of L. interrogans serovar Copenhageni strain Fiocruz L1-130. However, bacteria were recovered from the kidneys of all animals. Of eight animals immunized with LigB0-7, only three survived Leptospira challenge, one of which lacked renal colonization and had antibodies to native LigB by immunoblot. In addition, sera from two of the three LigB0-7 immunized survivors cross-reacted with LigA11-13, a region of LigA that is sufficient for protection. In summary, we confirmed that LigA7'-13 protects hamsters from death but not infection, and immunization with LigB0-7, either alone or in combination with LigA7'-13, did not confer sterilizing immunity.
Conflict of interest statement
Figures



Similar articles
-
Oral immunization with Escherichia coli expressing a lipidated form of LigA protects hamsters against challenge with Leptospira interrogans serovar Copenhageni.Infect Immun. 2014 Feb;82(2):893-902. doi: 10.1128/IAI.01533-13. Epub 2013 Dec 9. Infect Immun. 2014. PMID: 24478102 Free PMC article.
-
A LigA three-domain region protects hamsters from lethal infection by Leptospira interrogans.PLoS Negl Trop Dis. 2011 Dec;5(12):e1422. doi: 10.1371/journal.pntd.0001422. Epub 2011 Dec 13. PLoS Negl Trop Dis. 2011. PMID: 22180800 Free PMC article.
-
The terminal portion of leptospiral immunoglobulin-like protein LigA confers protective immunity against lethal infection in the hamster model of leptospirosis.Vaccine. 2007 Aug 14;25(33):6277-86. doi: 10.1016/j.vaccine.2007.05.053. Epub 2007 Jun 14. Vaccine. 2007. PMID: 17629368 Free PMC article.
-
Leptospiral Immunoglobulin-Like Domain Proteins: Roles in Virulence and Immunity.Front Immunol. 2021 Jan 8;11:579907. doi: 10.3389/fimmu.2020.579907. eCollection 2020. Front Immunol. 2021. PMID: 33488581 Free PMC article. Review.
-
Expansion of the in vitro assay for Leptospira potency testing to other serovars: case study with Leptospira Hardjo.Biologicals. 2013 Sep;41(5):323-4. doi: 10.1016/j.biologicals.2013.06.003. Epub 2013 Jul 6. Biologicals. 2013. PMID: 23838569 Review.
Cited by
-
Reduced Renal Colonization and Enhanced Protection by Leptospiral Factor H Binding Proteins as a Multisubunit Vaccine Against Leptospirosis in Hamsters.Vaccines (Basel). 2019 Aug 22;7(3):95. doi: 10.3390/vaccines7030095. Vaccines (Basel). 2019. PMID: 31443566 Free PMC article.
-
Role of animal models in biomedical research: a review.Lab Anim Res. 2022 Jul 1;38(1):18. doi: 10.1186/s42826-022-00128-1. Lab Anim Res. 2022. PMID: 35778730 Free PMC article. Review.
-
Anti-Leptospira immunoglobulin profiling in mice reveals strain specific IgG and persistent IgM responses associated with virulence and renal colonization.PLoS Negl Trop Dis. 2021 Mar 11;15(3):e0008970. doi: 10.1371/journal.pntd.0008970. eCollection 2021 Mar. PLoS Negl Trop Dis. 2021. PMID: 33705392 Free PMC article.
-
Leptospira Lipid A Is a Potent Adjuvant That Induces Sterilizing Immunity against Leptospirosis.Vaccines (Basel). 2023 Dec 6;11(12):1824. doi: 10.3390/vaccines11121824. Vaccines (Basel). 2023. PMID: 38140228 Free PMC article.
-
The interaction of two novel putative proteins of Leptospira interrogans with E-cadherin, plasminogen and complement components with potential role in bacterial infection.Virulence. 2019 Dec;10(1):734-753. doi: 10.1080/21505594.2019.1650613. Virulence. 2019. PMID: 31422744 Free PMC article.
References
-
- Adler B, de la Peña Moctezuma A. Leptospira and leptospirosis. Vet Microbiol. 2010; 140(3–4):287–96. doi: 10.1016/j.vetmic.2009.03.012 - DOI - PubMed
-
- Ko AI, Goarant C, Picardeau M. Leptospira: The dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol. 2009; 7(10):736–47. doi: 10.1038/nrmicro2208 - DOI - PMC - PubMed
-
- Evangelista KV, Coburn J. Leptospira as an emerging pathogen: a review of its biology, pathogenesis and host immune responses. Future Microbiol. 2010; 5(9):1413–25. doi: 10.2217/fmb.10.102 - DOI - PMC - PubMed
-
- Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296–326. doi: 10.1128/CMR.14.2.296-326.2001 - DOI - PMC - PubMed
-
- Athanazio DA, Silva EF, Santos CS, Rocha GM, Vannier-Santos MA, McBride AJA, et al. Rattus norvegicus as a model for persistent renal colonization by pathogenic Leptospira interrogans. Acta Tropica. 2008; 105(2):176–80. doi: 10.1016/j.actatropica.2007.10.012 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

