PA21, a novel phosphate binder, improves renal osteodystrophy in rats with chronic renal failure
- PMID: 28704404
- PMCID: PMC5509238
- DOI: 10.1371/journal.pone.0180430
PA21, a novel phosphate binder, improves renal osteodystrophy in rats with chronic renal failure
Abstract
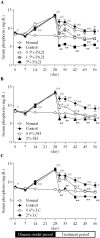
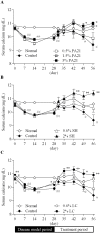
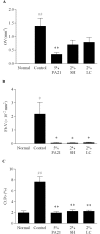
The effects of PA21, a novel iron-based and non-calcium-based phosphate binder, on hyperphosphatemia and its accompanying bone abnormality in chronic kidney disease-mineral and bone disorder (CKD-MBD) were evaluated. Rats with adenine-induced chronic renal failure (CRF) were prepared by feeding them an adenine-containing diet for four weeks. They were also freely fed a diet that contained PA21 (0.5, 1.5, and 5%), sevelamer hydrochloride (0.6 and 2%) or lanthanum carbonate hydrate (0.6 and 2%) for four weeks. Blood biochemical parameters were measured and bone histomorphometry was performed for femurs, which were isolated after drug treatment. Serum phosphorus and parathyroid hormone (PTH) levels were higher in the CRF rats. Administration of phosphate binders for four weeks decreased serum phosphorus and PTH levels in a dose-dependent manner and there were significant decreases in the AUC0-28 day of these parameters in 5% PA21, 2% sevelamer hydrochloride, and 2% lanthanum carbonate hydrate groups compared with that in the CRF control group. Moreover, osteoid volume improved significantly in 5% of the PA21 group, and fibrosis volume and cortical porosity were ameliorated in 5% PA21, 2% sevelamer hydrochloride, and 2% lanthanum carbonate hydrate groups. These results suggest that PA21 is effective against hyperphosphatemia, secondary hyperparathyroidism, and bone abnormalities in CKD-MBD as sevelamer hydrochloride and lanthanum carbonate hydrate are, and that PA21 is a new potential alternative to phosphate binders.
Conflict of interest statement
Figures



References
-
- Moe S, Drüeke T, Cunningham J, Goodman W, Martin K, Olgaard K, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006; 69: 1945–1953 doi: 10.1038/sj.ki.5000414 - DOI - PubMed
-
- Tonelli M, Pannu N, Manns B. Oral phosphate binders in patients with kidney failure. N Engl J Med. 2010; 362: 1312–1324 doi: 10.1056/NEJMra0912522 - DOI - PubMed
-
- Hruska KA, Saab G, Mathew S, Lund R. Renal osteodystrophy, phosphate homeostasis, and vascular calcification. Semin Dial. 2007; 20: 309–315 doi: 10.1111/j.1525-139X.2007.00300.x - DOI - PubMed
-
- Kanbay M, Goldsmith D, Akcay, Covic A. Phosphate—the silent stealthy cardiorenal culprit in all stages of chronic kidney disease: a systematic review. Blood Purif. 2009; 27: 220–230 doi: 10.1159/000197562 - DOI - PubMed
-
- Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001; 38: 938–942 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

