Streptococcus gallolyticus subsp. gallolyticus promotes colorectal tumor development
- PMID: 28704539
- PMCID: PMC5509344
- DOI: 10.1371/journal.ppat.1006440
Streptococcus gallolyticus subsp. gallolyticus promotes colorectal tumor development
Abstract
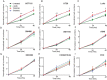
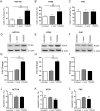
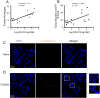
Streptococcus gallolyticus subsp. gallolyticus (Sg) has long been known to have a strong association with colorectal cancer (CRC). This knowledge has important clinical implications, and yet little is known about the role of Sg in the development of CRC. Here we demonstrate that Sg promotes human colon cancer cell proliferation in a manner that depends on cell context, bacterial growth phase and direct contact between bacteria and colon cancer cells. In addition, we observed increased level of β-catenin, c-Myc and PCNA in colon cancer cells following incubation with Sg. Knockdown or inhibition of β-catenin abolished the effect of Sg. Furthermore, mice administered with Sg had significantly more tumors, higher tumor burden and dysplasia grade, and increased cell proliferation and β-catenin staining in colonic crypts compared to mice receiving control bacteria. Finally, we showed that Sg is present in the majority of CRC patients and is preferentially associated with tumor compared to normal tissues obtained from CRC patients. These results taken together establish for the first time a tumor-promoting role of Sg that involves specific bacterial and host factors and have important clinical implications.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Cancer Facts & Figures 2013. American Cancer Society.
-
- Siegel R, Desantis C, Jemal A (2014) Colorectal cancer statistics, 2014. CA Cancer J Clin 64: 104–117. doi: 10.3322/caac.21220 - DOI - PubMed
-
- Boleij A, Tjalsma H (2012) Gut bacteria in health and disease: a survey on the interface between intestinal microbiology and colorectal cancer. Biol Rev Camb Philos Soc 87: 701–730. doi: 10.1111/j.1469-185X.2012.00218.x - DOI - PubMed
-
- Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, et al. (2013) Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 14: 207–215. doi: 10.1016/j.chom.2013.07.007 - DOI - PMC - PubMed
-
- Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, et al. (2013) Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe 14: 195–206. doi: 10.1016/j.chom.2013.07.012 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

