Diabetes Enhances IL-17 Expression and Alters the Oral Microbiome to Increase Its Pathogenicity
- PMID: 28704648
- PMCID: PMC5701758
- DOI: 10.1016/j.chom.2017.06.014
Diabetes Enhances IL-17 Expression and Alters the Oral Microbiome to Increase Its Pathogenicity
Abstract
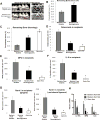
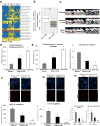
Diabetes is a risk factor for periodontitis, an inflammatory bone disorder and the greatest cause of tooth loss in adults. Diabetes has a significant impact on the gut microbiota; however, studies in the oral cavity have been inconclusive. By 16S rRNA sequencing, we show here that diabetes causes a shift in oral bacterial composition and, by transfer to germ-free mice, that the oral microbiota of diabetic mice is more pathogenic. Furthermore, treatment with IL-17 antibody decreases the pathogenicity of the oral microbiota in diabetic mice; when transferred to recipient germ-free mice, oral microbiota from IL-17-treated donors induced reduced neutrophil recruitment, reduced IL-6 and RANKL, and less bone resorption. Thus, diabetes-enhanced IL-17 alters the oral microbiota and renders it more pathogenic. Our findings provide a mechanistic basis to better understand how diabetes can increase the risk and severity of tooth loss.
Keywords: IL-17; bone loss; diabetes; dysbiosis; microbiota; osteoclast; pathogen; periodontitis.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures

References
-
- Anderson M. A new method for non-parametric multivariate analysis of variance. Austral Ecology. 2001:32–46.
-
- Camelo-Castillo A, Novoa L, Balsa-Castro C, Blanco J, Mira A, Tomas I. Relationship between periodontitis-associated subgingival microbiota and clinical inflammation by 16S pyrosequencing. J Clin Periodontol. 2015;42:1074–1082. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

