Fusion Stage of HIV-1 Entry Depends on Virus-Induced Cell Surface Exposure of Phosphatidylserine
- PMID: 28704658
- PMCID: PMC5558241
- DOI: 10.1016/j.chom.2017.06.012
Fusion Stage of HIV-1 Entry Depends on Virus-Induced Cell Surface Exposure of Phosphatidylserine
Abstract
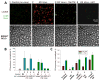
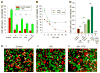
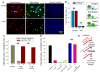
HIV-1 entry into host cells starts with interactions between the viral envelope glycoprotein (Env) and cellular CD4 receptors and coreceptors. Previous work has suggested that efficient HIV entry also depends on intracellular signaling, but this remains controversial. Here we report that formation of the pre-fusion Env-CD4-coreceptor complexes triggers non-apoptotic cell surface exposure of the membrane lipid phosphatidylserine (PS). HIV-1-induced PS redistribution depends on Ca2+ signaling triggered by Env-coreceptor interactions and involves the lipid scramblase TMEM16F. Externalized PS strongly promotes Env-mediated membrane fusion and HIV-1 infection. Blocking externalized PS or suppressing TMEM16F inhibited Env-mediated fusion. Exogenously added PS promoted fusion, with fusion dependence on PS being especially strong for cells with low surface density of coreceptors. These findings suggest that cell-surface PS acts as an important cofactor that promotes the fusogenic restructuring of pre-fusion complexes and likely focuses the infection on cells conducive to PS signaling.
Keywords: HIV entry; TMEM16F activity; cell activation; cell signaling; gp120-CD4-coreceptor; hemifusion; lipid scramblase; membrane fusion; phosphatidylserine exposure; viral entry.
Published by Elsevier Inc.
Figures




Comment in
-
Viral infection: When two become one.Nat Rev Microbiol. 2017 Sep;15(9):511. doi: 10.1038/nrmicro.2017.85. Epub 2017 Jul 24. Nat Rev Microbiol. 2017. PMID: 28736446 No abstract available.
References
-
- Baur A, Garber S, Peterlin BM. Effects of CD45 on NF-kappa B. Implications for replication of HIV-1. J Immunol. 1994;152:976–983. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

