The impact of antiretroviral therapy on symptom burden among HIV outpatients with low CD4 count in rural Uganda: nested longitudinal cohort study
- PMID: 28705181
- PMCID: PMC5508714
- DOI: 10.1186/s12904-017-0215-y
The impact of antiretroviral therapy on symptom burden among HIV outpatients with low CD4 count in rural Uganda: nested longitudinal cohort study
Erratum in
-
Erratum to: BMC Palliative Care, Vol. 17.BMC Palliat Care. 2017 Oct 10;16(1):51. doi: 10.1186/s12904-017-0233-9. BMC Palliat Care. 2017. PMID: 29017489 Free PMC article. No abstract available.
Abstract
Background: Individuals with HIV have a high prevalence of physical and psychological symptoms throughout their disease course. Despite the clinical and public health implications of unresolved pain and symptoms, little is known about the effect of anti-retroviral therapy (ART) on these outcomes. This study aimed to assess the impact on symptom burden for the year after ART initiation in individuals with a CD4 count <200 cells/uL in Uganda.
Methods: HIV-infected, ART-naıve adults referred from voluntary testing and counseling services in rural Uganda for enrollment into a randomized controlled trial to test fluconazole as primary prophylaxis against cryptococcal disease were invited to complete the Memorial Symptom Assessment Scale-Short Form (MSAS-SF) prior to commencing ART and at two subsequent follow up visits. This tool measures self-reported 7-day period prevalence and associated burden of physical and psychological symptoms. Changes in the total number of symptoms and distress indices with time on ART and trial arm were investigated through fitting Linear Mixed Models for repeated measures.
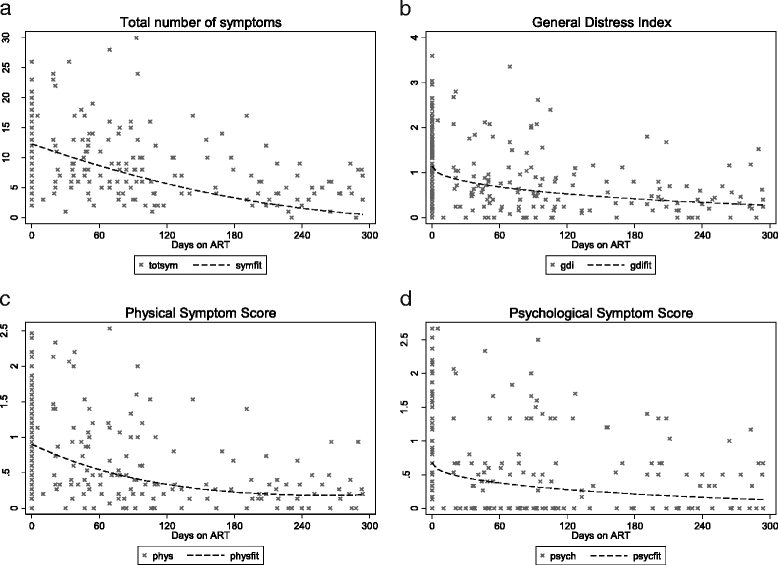
Results: During the first year of ART initiation the prevalence of most individual symptoms remained constant. The notable exceptions which improved after commencing ART are as follow; prevalence of pain (prevalence changed from 79% to 60%), weight loss (67% to 31%), lack of appetite (46% to 28%), feeling sad (52% to 25%) and difficulty sleeping (35% to 23%). The total number of symptoms and distress indices reduced after treatment commenced. Of concern was that half or more study participants remained with symptoms of pain (60%), itching (57%), skin changes (53%) and numbness in hands and feet (52%) after starting ART. Sixteen symptoms remained with a burden of 25% or more.
Conclusion: Despite the beneficial effect of ART on reducing symptoms, some patients continue to experience a high symptom burden. It is essential that HIV services in sub-Saharan Africa integrate management of symptoms into their programmes.
Trial registration: CRYPTOPRO [ISRCTN 76481529 ], November 2004.
Keywords: ART; Africa; HIV; Pain; Palliative care; Symptom burden; Symptom control.
Conflict of interest statement
Ethics approval and consent to participate
This study received ethical approval from the Uganda Virus Research Institute and Uganda National Council for Science and Technology. Written informed consent was obtained from all particpants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Global AIDS Update 2016. [http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update...]. Accessed July 2017.
-
- Harding R, Simms V, Penfold S, Downing J, Namisango E, Powell RA, Mwangi-Powell F, Moreland S, Gikaara N, Atieno M, et al. Quality of life and wellbeing among HIV outpatients in East Africa: a multicentre observational study. BMC Infect Dis. 2014;14:613. doi: 10.1186/s12879-014-0613-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials