The M-phase specific hyperphosphorylation of Staufen2 involved the cyclin-dependent kinase CDK1
- PMID: 28705199
- PMCID: PMC5513041
- DOI: 10.1186/s12860-017-0142-z
The M-phase specific hyperphosphorylation of Staufen2 involved the cyclin-dependent kinase CDK1
Erratum in
-
Correction to: The M-phase specific hyperphosphorylation of Staufen2 involved the cyclin-dependent kinase CDK1.BMC Cell Biol. 2018 Sep 10;19(1):20. doi: 10.1186/s12860-018-0171-2. BMC Cell Biol. 2018. PMID: 30200875 Free PMC article.
Abstract
Background: Staufen2 (STAU2) is an RNA-binding protein involved in the post-transcriptional regulation of gene expression. This protein was shown to be required for organ formation and cell differentiation. Although STAU2 functions have been reported in neuronal cells, its role in dividing cells remains deeply uncharacterized. Especially, its regulation during the cell cycle is completely unknown.
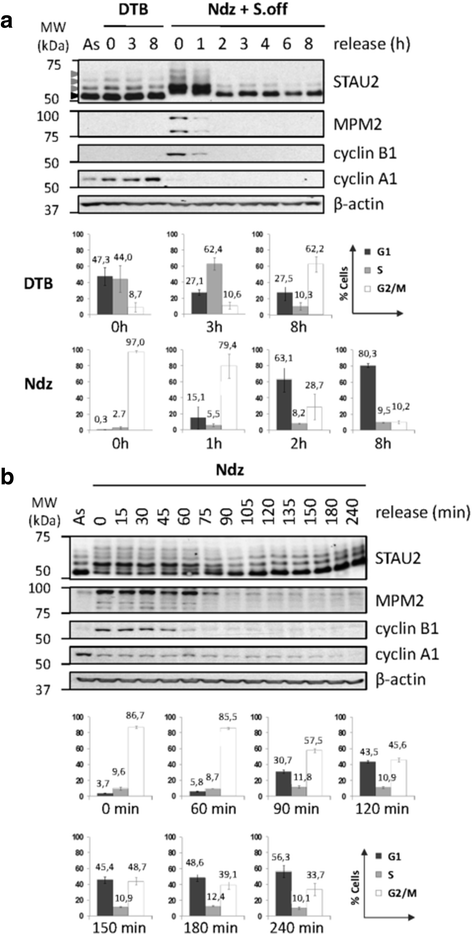
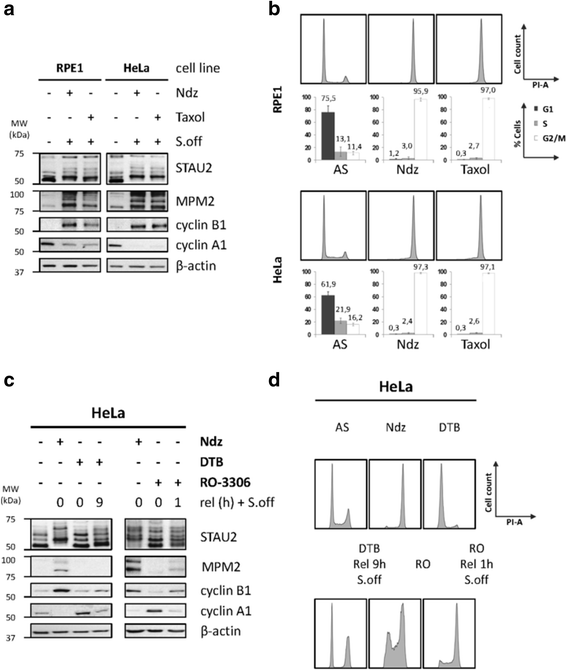
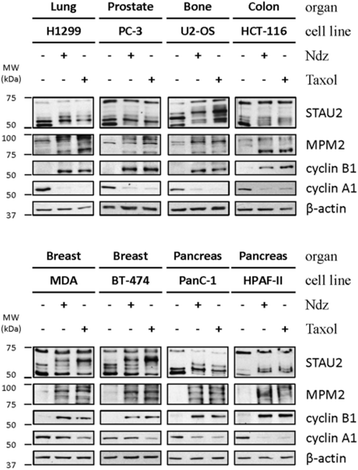
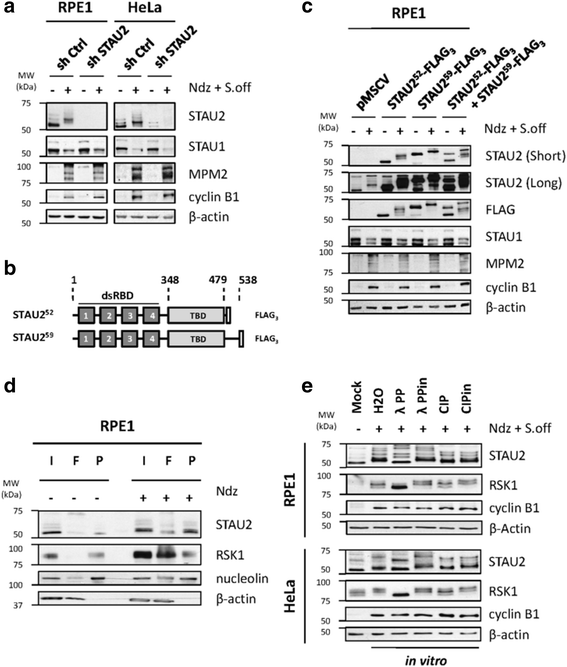
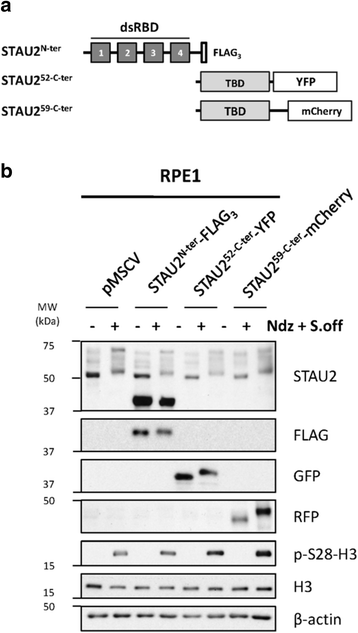
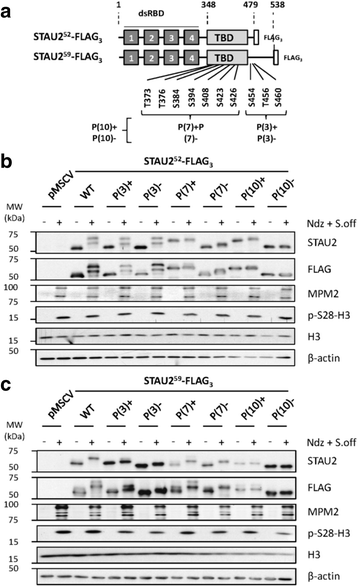
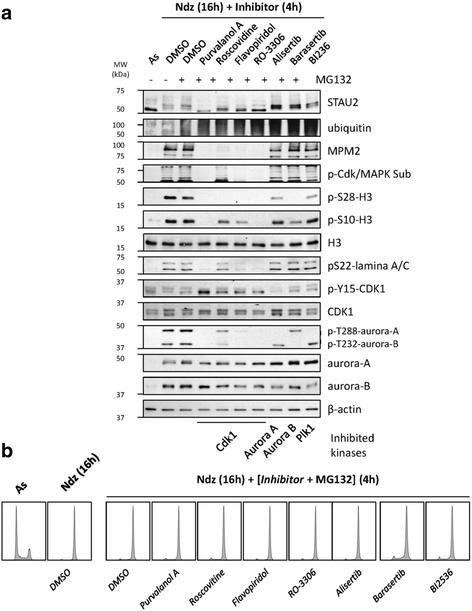
Results: In this study, we showed that STAU2 isoforms display a mitosis-specific slow migration pattern on SDS-gels in all tested transformed and untransformed cell lines. Deeper analyses in hTert-RPE1 and HeLa cells further indicated that the slow migration pattern of STAU2 isoforms is due to phosphorylation. Time course studies showed that STAU2 phosphorylation occurs before prometaphase and terminates as cells exit mitosis. Interestingly, STAU2 isoforms were phosphorylated on several amino acid residues in the C-terminal half via the cyclin-dependent kinase 1 (Cdk1), an enzyme known to play crucial roles during mitosis. Introduction of phospho-mimetic or phospho-null mutations in STAU2 did not impair its RNA-binding capacity, its stability, its interaction with protein co-factors or its sub-cellular localization, suggesting that STAU2 phosphorylation in mitosis does not regulate these functions. Similarly, STAU2 phosphorylation is not likely to be crucial for cell cycle progression since expression of phosphorylation mutants in hTert-RPE1 cells did not impair cell proliferation.
Conclusions: Altogether, these results indicate that STAU2 isoforms are phosphorylated during mitosis and that the phosphorylation process involves Cdk1. The meaning of this post-translational modification is still elusive.
Keywords: Cell cycle; Cyclin-dependent kinase; Mitosis; Phosphorylation; RNA-binding protein; Staufen2.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

