Impact of Enteroviral Polymerase Chain Reaction Testing on Length of Stay for Infants 60 Days Old or Younger
- PMID: 28705656
- PMCID: PMC5870831
- DOI: 10.1016/j.jpeds.2017.06.021
Impact of Enteroviral Polymerase Chain Reaction Testing on Length of Stay for Infants 60 Days Old or Younger
Abstract
Objective: To determine the impact of a cerebrospinal fluid enterovirus polymerase chain reaction (PCR) test performance on hospital length of stay (LOS) in a large multicenter cohort of infants undergoing evaluation for central nervous system infection.
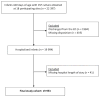
Study design: We performed a planned secondary analysis of a retrospective cohort of hospitalized infants ≤60 days of age who had a cerebrospinal fluid culture obtained at 1 of 18 participating centers (2005-2013). After adjustment for patient age and study year as well as clustering by hospital center, we compared LOS for infants who had an enterovirus PCR test performed vs not performed and among those tested, for infants with a positive vs negative test result.
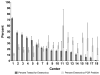
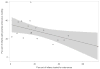
Results: Of 19 953 hospitalized infants, 4444 (22.3%) had an enterovirus PCR test performed and 945 (21.3% of tested infants) had positive test results. Hospital LOS was similar for infants who had an enterovirus PCR test performed compared with infants who did not (incident rate ratio 0.98 hours; 95% CI 0.89-1.06). However, infants PCR positive for enterovirus had a 38% shorter LOS than infants PCR negative for enterovirus (incident rate ratio 0.62 hours; 95% CI 0.57-0.68). No infant with a positive enterovirus PCR test had bacterial meningitis (0%; 95% CI 0-0.4).
Conclusions: Although enterovirus PCR testing was not associated with a reduction in LOS, infants with a positive enterovirus PCR test had a one-third shorter LOS compared with infants with a negative enterovirus PCR test. Focused enterovirus PCR test use could increase the impact on LOS for infants undergoing cerebrospinal fluid evaluation.
Keywords: enterovirus; meningitis; neonate; young infant.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Comment in
-
What are the benefits of molecular testing of cerebrospinal fluid of young febrile infants for enteroviruses?J Pediatr. 2017 Oct;189:1. doi: 10.1016/j.jpeds.2017.08.019. J Pediatr. 2017. PMID: 28947051 No abstract available.
References
-
- Aronson PL, Thurm C, Alpern ER, Alessandrini EA, Williams DJ, Shah SS, et al. Variation in Care of the Febrile Young Infant <90 Days in US Pediatric Emergency Departments. Pediatrics. 2014;134:667–77. - PubMed
-
- Levine DA, Platt SL, Dayan PS, Macias CG, Zorc JJ, Krief W, et al. Risk of serious bacterial infection in young febrile infants with respiratory syncytial virus infections. Pediatrics. 2004;113:1728–34. - PubMed
-
- Baker MD, Avner JR, Bell LM. Failure of infant observation scales in detecting serious illness in febrile, 4- to 8-week-old infants. Pediatrics. 1990;85:1040–43. - PubMed
-
- Byington CL, Taggart EW, Carroll KC, Hillyard DR. A polymerase chain reaction-based epidemiologic investigation of the incidence of nonpolio enteroviral infections in febrile and afebrile infants 90 days and younger. Pediatrics. 1999;103:E27. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources