Augmentation of cGMP/PKG pathway and colonic motility by hydrogen sulfide
- PMID: 28705807
- PMCID: PMC5668569
- DOI: 10.1152/ajpgi.00161.2017
Augmentation of cGMP/PKG pathway and colonic motility by hydrogen sulfide
Abstract
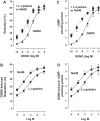
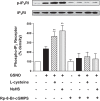
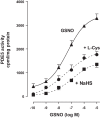
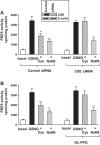
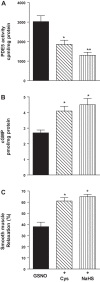
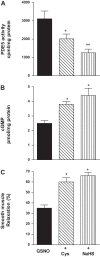
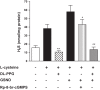
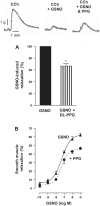
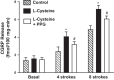
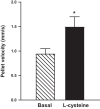
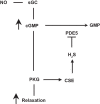
Hydrogen sulfide (H2S), like nitric oxide (NO), causes smooth muscle relaxation, but unlike NO, does not stimulate soluble guanylyl cyclase (sGC) activity and generate cyclic guanosine 5'-monophosphate (cGMP). The aim of this study was to investigate the interplay between NO and H2S in colonic smooth muscle. In colonic smooth muscle from rabbit, mouse, and human, l-cysteine, substrate of cystathionine-γ-lyase (CSE), or NaHS, an H2S donor, inhibited phosphodiesterase 5 (PDE5) activity and augmented the increase in cGMP levels, IP3 receptor phosphorylation at Ser1756 (measured as a proxy for PKG activation), and muscle relaxation in response to NO donor S-nitrosoglutathione (GSNO), suggesting augmentation of cGMP/PKG pathway by H2S. The inhibitory effect of l-cysteine, but not NaHS, on PDE5 activity was blocked in cells transfected with CSE siRNA or treated with CSE inhibitor d,l-propargylglycine (dl-PPG), suggesting activation of CSE and generation of H2S in response to l-cysteine. H2S levels were increased in response to l-cysteine, and the effect of l-cysteine was augmented by GSNO in a cGMP-dependent protein kinase-sensitive manner, suggesting augmentation of CSE/H2S by cGMP/PKG pathway. As a result, GSNO-induced relaxation was inhibited by dl-PPG. In flat-sheet preparation of colon, l-cysteine augmented calcitonin gene-related peptide release in response to mucosal stimulation, and in intact segments, l-cysteine increased the velocity of pellet propulsion. These results demonstrate that in colonic smooth muscle, there is a novel interplay between NO and H2S. NO generates H2S via cGMP/PKG pathway, and H2S, in turn, inhibits PDE5 activity and augments NO-induced cGMP levels. In the intact colon, H2S promotes colonic transit.NEW & NOTEWORTHY Hydrogen sulfide (H2S) and nitric oxide (NO) are important regulators of gastrointestinal motility. The studies herein provide the cross talk between NO and H2S signaling to mediate smooth muscle relaxation and colonic transit. H2S inhibits phosphodiesterase 5 activity to augment cGMP levels in response to NO, which, in turn, via cGMP/PKG pathway, generates H2S. These studies suggest that interventions targeted at restoring NO and H2S homeostasis within the smooth muscle may provide novel therapeutic approaches to mitigate motility disorders.
Keywords: muscle relaxation; nitric oxide; phosphodiesterase 5; signaling.
Copyright © 2017 the American Physiological Society.
Figures
Similar articles
-
Inhibition of RhoA-dependent pathway and contraction by endogenous hydrogen sulfide in rabbit gastric smooth muscle cells.Am J Physiol Cell Physiol. 2015 Mar 15;308(6):C485-95. doi: 10.1152/ajpcell.00280.2014. Epub 2015 Jan 7. Am J Physiol Cell Physiol. 2015. PMID: 25567809 Free PMC article.
-
Inhibition of RhoA/Rho kinase pathway and smooth muscle contraction by hydrogen sulfide.Pharmacol Res Perspect. 2017 Oct;5(5):e00343. doi: 10.1002/prp2.343. Pharmacol Res Perspect. 2017. PMID: 28971603 Free PMC article.
-
Cardioprotection by H2S engages a cGMP-dependent protein kinase G/phospholamban pathway.Cardiovasc Res. 2015 Jun 1;106(3):432-42. doi: 10.1093/cvr/cvv129. Epub 2015 Apr 13. Cardiovasc Res. 2015. PMID: 25870184 Free PMC article.
-
Role of cGMP in hydrogen sulfide signaling.Nitric Oxide. 2015 Apr 30;46:7-13. doi: 10.1016/j.niox.2014.12.004. Epub 2014 Dec 29. Nitric Oxide. 2015. PMID: 25553675 Review.
-
Nitric oxide-cyclic GMP pathway with some emphasis on cavernosal contractility.Int J Impot Res. 2004 Dec;16(6):459-69. doi: 10.1038/sj.ijir.3901256. Int J Impot Res. 2004. PMID: 15229623 Review.
Cited by
-
Mechanism of Oxytocin-Induced Contraction in Rat Gastric Circular Smooth Muscle.Int J Mol Sci. 2022 Dec 27;24(1):441. doi: 10.3390/ijms24010441. Int J Mol Sci. 2022. PMID: 36613886 Free PMC article.
-
Role of hydrogen sulfide in health and disease.MedComm (2020). 2024 Aug 16;5(9):e661. doi: 10.1002/mco2.661. eCollection 2024 Sep. MedComm (2020). 2024. PMID: 39156767 Free PMC article. Review.
-
Gasotransmitters in pregnancy: from conception to uterine involution.Biol Reprod. 2019 Jul 1;101(1):4-25. doi: 10.1093/biolre/ioz038. Biol Reprod. 2019. PMID: 30848786 Free PMC article. Review.
-
Gasotransmitters in the tumor microenvironment: Impacts on cancer chemotherapy (Review).Mol Med Rep. 2022 Jul;26(1):233. doi: 10.3892/mmr.2022.12749. Epub 2022 May 26. Mol Med Rep. 2022. PMID: 35616143 Free PMC article. Review.
-
Nitric oxide and ion channels mediate L-cysteine-induced inhibition of colonic smooth muscle contraction.J Muscle Res Cell Motil. 2024 Mar;45(1):11-20. doi: 10.1007/s10974-023-09664-2. Epub 2023 Dec 23. J Muscle Res Cell Motil. 2024. PMID: 38141146
References
-
- Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev 75: 725–748, 1995. - PubMed
-
- Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Zaid A, Giannogonas P, Cantalupo A, Dhayade S, Karalis KP, Wang R, Feil R, Cirino G. cGMP-dependent protein kinase contributes to hydrogen sulfide-stimulated vasorelaxation. PLoS One 7: e53319, 2012. doi:10.1371/journal.pone.0053319. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

