Biodistribution and Dosimetry of 18F-Meta-Fluorobenzylguanidine: A First-in-Human PET/CT Imaging Study of Patients with Neuroendocrine Malignancies
- PMID: 28705916
- PMCID: PMC5750519
- DOI: 10.2967/jnumed.117.193169
Biodistribution and Dosimetry of 18F-Meta-Fluorobenzylguanidine: A First-in-Human PET/CT Imaging Study of Patients with Neuroendocrine Malignancies
Abstract
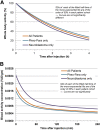
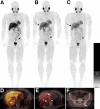
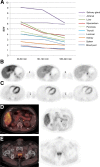
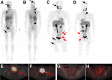
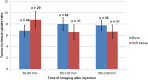
123I-meta-iodobenzylguanidine (123I-MIBG) imaging is currently a mainstay in the evaluation of many neuroendocrine tumors, especially neuroblastoma. 123I-MIBG imaging has several limitations that can be overcome by the use of a PET agent. 18F-meta-fluorobenzylguanidine (18F-MFBG) is a PET analog of MIBG that may allow for single-day, high-resolution quantitative imaging. We conducted a first-in-human study of 18F-MFBG PET imaging to evaluate the safety, feasibility, pharmacokinetics, and dosimetry of 18F-MFBG in neuroendocrine tumors (NETs). Methods: Ten patients (5 with neuroblastoma and 5 with paraganglioma/pheochromocytoma) received 148-444 MBq (4-12mCi) of 18F-MFBG intravenously followed by serial whole-body imaging at 0.5-1, 1-2, and 3-4 after injection. Serial blood samples (a total of 6) were also obtained starting at 5 min after injection to as late as 4 h after injection; whole-body distribution and blood clearance data, lesion uptake, and normal-tissue uptake were determined, and radiation-absorbed doses to normal organs were calculated using OLINDA. Results: No side effects were seen in any patient after 18F-MFBG injection. Tracer distribution showed prominent activity in the blood pool, liver, and salivary glands that decreased with time. Mild uptake was seen in the kidneys and spleen, which also decreased with time. Urinary excretion was prominent, with an average of 45% of the administered activity in the bladder by 1 h after injection; whole-body clearance was monoexponential, with a mean biologic half-life of 1.95 h, whereas blood clearance was biexponential, with a mean biologic half-life of 0.3 h (58%) for the rapid α phase and 6.1 h (42%) for the slower β phase. The urinary bladder received the highest radiation dose with a mean absorbed dose of 0.186 ± 0.195 mGy/MBq. The mean total-body dose was 0.011 ± 0.011 mGy/MBq, and the effective dose was 0.023 ± 0.012 mSv/MBq. Both skeletal and soft-tissue lesions were visualized with high contrast. The SUVmax (mean ± SD ) of lesions at 1-2 h after injection was 8.6 ± 9.6. Conclusion: Preliminary data show that 18F-MFBG imaging is safe and has favorable biodistribution and kinetics with good targeting of lesions. PET imaging with 18F-MFBG allows for same-day imaging of NETs. 18F-MFBG appears highly promising for imaging of patients with NETs, especially children with neuroblastoma.
Keywords: 18F-MFBG; MIBG; dosimetry; neuroblastoma; neuroendocrine.
© 2018 by the Society of Nuclear Medicine and Molecular Imaging.
Figures
Comment in
-
[18F] MFBG PET imaging: biodistribution, pharmacokinetics, and comparison with [123I] MIBG in neural crest tumour patients.Eur J Nucl Med Mol Imaging. 2023 Mar;50(4):1134-1145. doi: 10.1007/s00259-022-06046-7. Epub 2022 Nov 26. Eur J Nucl Med Mol Imaging. 2023. PMID: 36435928
References
-
- Decarolis B, Schneider C, Hero B, et al. Iodine-123 metaiodobenzylguanidine scintigraphy scoring allows prediction of outcome in patients with stage 4 neuroblastoma: results of the Cologne interscore comparison study. J Clin Oncol. 2013;31:944–951. - PubMed
-
- Bombardieri E, Giammarile F, Aktolun C, et al. 131I/123I-metaiodobenzylguanidine (mIBG) scintigraphy: procedure guidelines for tumour imaging. Eur J Nucl Med Mol Imaging. 2010;37:2436–2446. - PubMed
-
- Boubaker A, Bischof Delaloye A. MIBG scintigraphy for the diagnosis and follow-up of children with neuroblastoma. Q J Nucl Med Mol Imaging. 2008;52:388–402. - PubMed
-
- Katzenstein HM, Cohn SL, Shore RM, et al. Scintigraphic response by 123I-metaiodobenzylguanidine scan correlates with event-free survival in high-risk neuroblastoma. J Clin Oncol. 2004;22:3909–3915. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
