Curative Multicycle Radioimmunotherapy Monitored by Quantitative SPECT/CT-Based Theranostics, Using Bispecific Antibody Pretargeting Strategy in Colorectal Cancer
- PMID: 28705917
- PMCID: PMC5666642
- DOI: 10.2967/jnumed.117.193250
Curative Multicycle Radioimmunotherapy Monitored by Quantitative SPECT/CT-Based Theranostics, Using Bispecific Antibody Pretargeting Strategy in Colorectal Cancer
Abstract
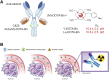
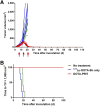
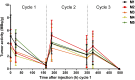
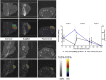
Radioimmunotherapy of solid tumors using antibody-targeted radionuclides has been limited by low therapeutic indices (TIs). We recently reported a novel 3-step pretargeted radioimmunotherapy (PRIT) strategy based on a glycoprotein A33 (GPA33)-targeting bispecific antibody and a small-molecule radioactive hapten, a complex of 177Lu and S-2-(4-aminobenzyl)-1,4,7,10-tetraazacyclododecane tetraacetic acid (177Lu-DOTA-Bn), that leads to high TIs for radiosensitive tissues such as blood (TI = 73) and kidney (TI = 12). We tested our hypothesis that a fractionated anti-GPA33 DOTA-PRIT regimen calibrated to deliver a radiation absorbed dose to tumor of more than 100 Gy would lead to a high probability of tumor cure while being well tolerated by nude mice bearing subcutaneous GPA33-positive SW1222 xenografts. Methods: We treated groups of nude mice bearing 7-d-old SW1222 xenografts with a fractionated 3-cycle anti-GPA33 DOTA-PRIT regimen (total administered 177Lu-DOTA-Bn activity, 167 MBq/mouse; estimated radiation absorbed dose to tumor, 110 Gy). In randomly selected mice undergoing treatment, serial SPECT/CT imaging was used to monitor treatment response and calculate radiation absorbed doses to tumor. Necropsy was done on surviving animals 100-200 d after treatment to determine frequency of cure and assess select normal tissues for treatment-related histopathologies. Results: Rapid exponential tumor progression was observed in control treatment groups (i.e., no treatment or 177Lu-DOTA-Bn only), leading to euthanasia due to excessive tumor burden, whereas 10 of 10 complete responses were observed for the DOTA-PRIT-treated animals within 30 d. Treatment was well tolerated, and 100% histologic cure was achieved in 9 of 9 assessable animals without detectable radiation damage to critical organs, including bone marrow and kidney. Radiation absorbed doses to tumor derived from SPECT/CT (102 Gy) and from biodistribution (110 Gy) agreed to within 6.9%. Of the total dose of approximately 100 Gy, the first dose contributes 30%, the second dose 60%, and the third dose 10%. Conclusion: In a GPA33-positive human colorectal cancer xenograft mouse model, we validated a SPECT/CT-based theranostic PRIT regimen that led to 100% complete responses and 100% cures without any treatment-related toxicities, based on high TIs for radiosensitive tissues. These studies support the view that anti-GPA33 DOTA-PRIT will be a potent radioimmunotherapy regimen for GPA33-positive colorectal cancer tumors in humans.
Keywords: SPECT; colorectal cancer; pretargeting; radioimmunotherapy.
© 2017 by the Society of Nuclear Medicine and Molecular Imaging.
Figures

Similar articles
-
Theranostic pretargeted radioimmunotherapy of internalizing solid tumor antigens in human tumor xenografts in mice: Curative treatment of HER2-positive breast carcinoma.Theranostics. 2018 Oct 6;8(18):5106-5125. doi: 10.7150/thno.26585. eCollection 2018. Theranostics. 2018. PMID: 30429889 Free PMC article.
-
GPA33-pretargeted radioimmunotherapy with mono- and bivalent DOTA-based Lu-177-labeled radiohaptens in a mouse orthotopic liver xenograft model of metastatic human colorectal cancer.Theranostics. 2025 May 25;15(13):6274-6289. doi: 10.7150/thno.107209. eCollection 2025. Theranostics. 2025. PMID: 40521192 Free PMC article.
-
Theranostic GPA33-Pretargeted Radioimmunotherapy of Human Colorectal Carcinoma with a Bivalent 177Lu-Labeled Radiohapten.J Nucl Med. 2024 Oct 1;65(10):1611-1618. doi: 10.2967/jnumed.124.267685. J Nucl Med. 2024. PMID: 39168519 Free PMC article.
-
Somatostatin receptor-targeted radionuclide therapy of tumors: preclinical and clinical findings.Semin Nucl Med. 2002 Apr;32(2):133-40. doi: 10.1053/snuc.2002.31027. Semin Nucl Med. 2002. PMID: 11965608 Review.
-
Recombinant bispecific monoclonal antibodies prepared by the dock-and-lock strategy for pretargeted radioimmunotherapy.Semin Nucl Med. 2010 May;40(3):190-203. doi: 10.1053/j.semnuclmed.2009.12.002. Semin Nucl Med. 2010. PMID: 20350628 Free PMC article. Review.
Cited by
-
Delivery of polymeric nanostars for molecular imaging and endoradiotherapy through the enhanced permeability and retention (EPR) effect.Theranostics. 2020 Jan 1;10(2):567-584. doi: 10.7150/thno.36777. eCollection 2020. Theranostics. 2020. PMID: 31903138 Free PMC article.
-
Prognostic evaluation of a multi-target magnetic bead-enriched circulating tumor cell-enriched identification system for colorectal cancer.J Gastrointest Oncol. 2024 Feb 29;15(1):134-146. doi: 10.21037/jgo-23-735. Epub 2024 Feb 28. J Gastrointest Oncol. 2024. PMID: 38482239 Free PMC article.
-
Therapeutic Applications of Pretargeting.Pharmaceutics. 2019 Sep 1;11(9):434. doi: 10.3390/pharmaceutics11090434. Pharmaceutics. 2019. PMID: 31480515 Free PMC article. Review.
-
Recent therapeutics in hepatocellular carcinoma.Am J Cancer Res. 2023 Jan 15;13(1):261-275. eCollection 2023. Am J Cancer Res. 2023. PMID: 36777510 Free PMC article. Review.
-
Development of a Tetravalent Anti-GPA33/Anti-CD3 Bispecific Antibody for Colorectal Cancers.Mol Cancer Ther. 2018 Oct;17(10):2164-2175. doi: 10.1158/1535-7163.MCT-18-0026. Epub 2018 Aug 6. Mol Cancer Ther. 2018. PMID: 30082472 Free PMC article.
References
-
- Scott AM, Lee FT, Jones R, et al. A phase I trial of humanized monoclonal antibody A33 in patients with colorectal carcinoma: biodistribution, pharmacokinetics, and quantitative tumor uptake. Clin Cancer Res. 2005;11:4810–4817. - PubMed
-
- Chong G, Lee FT, Hopkins W, et al. Phase I trial of 131I-huA33 in patients with advanced colorectal carcinoma. Clin Cancer Res. 2005;11:4818–4826. - PubMed
-
- Reardan DT, Meares CF, Goodwin DA, et al. Antibodies against metal chelates. Nature. 1985;316:265–268. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
