Improving vaccine trials in infectious disease emergencies
- PMID: 28706038
- PMCID: PMC5568786
- DOI: 10.1126/science.aam8334
Improving vaccine trials in infectious disease emergencies
Abstract
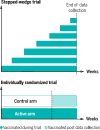
Unprecedented global effort is under way to facilitate the testing of countermeasures in infectious disease emergencies. Better understanding of the various options for trial design is needed in advance of outbreaks, as is preliminary global agreement on the most suitable designs for the various scenarios. What would enhance the speed, validity, and ethics of clinical studies of such countermeasures? Focusing on studies of vaccine efficacy and effectiveness in emergencies, we highlight three needs: for formal randomized trials-even in most emergencies; for individually randomized trials-even in many emergencies; and for six areas of innovation in trial methodology. These needs should inform current updates of protocols and roadmaps.
Copyright © 2017, American Association for the Advancement of Science.
Figures

References
-
- Cohen J, Kupferschmidt K. Tough choices ahead in Ebola vaccine trials. Science Magazine. 2014 - PubMed
-
- Cohen J. Issues continue to dog the testing of Ebola drugs and vaccines. Science Magazine. 2014
-
- WHO, editor. WHO. R&D Blueprint Update. Vol. 2017 Geneva: 2017.
-
- E. National Academies of Sciences, and Medicine. Integrating Clinical Research into Epidemic Response: The Ebola Experience. Washington, DC: 2017. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

