CCL11, a novel mediator of inflammatory bone resorption
- PMID: 28706221
- PMCID: PMC5509729
- DOI: 10.1038/s41598-017-05654-w
CCL11, a novel mediator of inflammatory bone resorption
Abstract
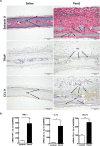
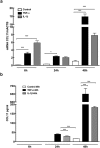
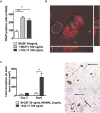
Normal bone homeostasis, which is regulated by bone-resorbing osteoclasts and bone-forming osteoblasts is perturbed by inflammation. In chronic inflammatory disease with disturbed bone remodelling, e.g. rheumatoid arthritis, patients show increased serum levels of the chemokine eotaxin-1 (CCL11). Herein, we demonstrate an inflammatory driven expression of CCL11 in bone tissue and a novel role of CCL11 in osteoclast migration and resorption. Using an inflammatory bone lesion model and primary cell cultures, we discovered that osteoblasts express CCL11 in vivo and in vitro and that expression increased during inflammatory conditions. Osteoclasts did not express CCL11, but the high affinity receptor CCR3 was significantly upregulated during osteoclast differentiation and found to colocalise with CCL11. Exogenous CCL11 was internalised in osteoclast and stimulated the migration of pre-osteoclast and concomitant increase in bone resorption. Our data pinpoints that the CCL11/CCR3 pathway could be a new target for treatment of inflammatory bone resorption.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

