Suppressed autophagic response underlies augmentation of renal ischemia/reperfusion injury by type 2 diabetes
- PMID: 28706237
- PMCID: PMC5509657
- DOI: 10.1038/s41598-017-05667-5
Suppressed autophagic response underlies augmentation of renal ischemia/reperfusion injury by type 2 diabetes
Abstract
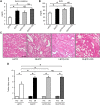
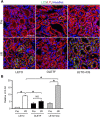
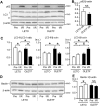
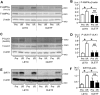
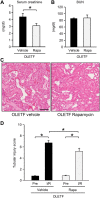
Diabetes mellitus is a major risk factor for acute kidney injury (AKI). Here, we hypothesized that suppression of autophagic response underlies aggravation of renal ischemia/reperfusion (I/R) injury by type 2 diabetes mellitus (T2DM). In OLETF, a rat model of T2DM, and its non-diabetic control, LETO, AKI was induced by unilateral nephrectomy and 30-min occlusion and 24-h reperfusion of the renal artery in the contralateral kidney. Levels of serum creatinine and blood urea nitrogen and tubular injury score after I/R were significantly higher in OLETF than in LETO. Administration of chloroquine, a widely used autophagy inhibitor, aggravated I/R-induced renal injury in LETO, but not in OLETF. In contrast to LETO, OLETF exhibited no increase in autophagosomes in the proximal tubules after I/R. Immunoblotting showed that I/R activated the AMPK/ULK1 pathway in LETO but not in OLETF, and mTORC1 activation after I/R was enhanced in OLETF. Treatment of OLETF with rapamycin, an mTORC1 inhibitor, partially restored autophagic activation in response to I/R and significantly attenuated I/R-induced renal injury. Collectively, these findings indicate that suppressed autophagic activation in proximal tubules by impaired AMPK/ULK1 signaling and upregulated mTORC1 activation underlies T2DM-induced worsening of renal I/R injury.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

