Connexin 50 Functions as an Adhesive Molecule and Promotes Lens Cell Differentiation
- PMID: 28706245
- PMCID: PMC5509658
- DOI: 10.1038/s41598-017-05647-9
Connexin 50 Functions as an Adhesive Molecule and Promotes Lens Cell Differentiation
Abstract
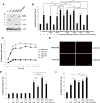
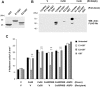
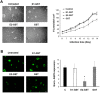
Connexins play essential roles in lens homeostasis and development. Here, we identified a new role for Cx50 that mediates cell-cell adhesion function. Cx50 enhanced the adhesive capability of AQP0. Interestingly, the expression of Cx50 alone promoted cell adhesion at a comparable level to AQP0; however, this cell adhesive function was not observed with other lens connexins, Cx43 and Cx46. Moreover, the adhesive property occurred in both homotypic with Cx50 expressed in both pairing cells and heterotypic with Cx50 in only one pairing cell, and this function appears to be unrelated to its role in forming gap junction channels. Cx50 KO lenses exhibited increased intercellular spaces between lens fiber cells. The second extracellular loop domain (E2) is primarily responsible for this adhesive function. Treatment with a fusion protein containing E2 domain inhibited cell adhesion. Furthermore, disruption of cell adhesion by the E2 domains impaired primary lens cell differentiation. Five critical amino acid residues in the E2 domain primarily are involved in cell adhesive function as well as lens epithelial-fiber differentiation. Together, these results suggest that in addition to forming gap junction channels, Cx50 acts as an adhesive molecule that is critical in maintaining lens fiber integrity and epithelial-fiber differentiation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
The second extracellular domain of connexin 50 is important for in cell adhesion, lens differentiation, and adhesion molecule expression.J Biol Chem. 2023 Mar;299(3):102965. doi: 10.1016/j.jbc.2023.102965. Epub 2023 Feb 1. J Biol Chem. 2023. PMID: 36736424 Free PMC article.
-
Aquaporin 0 enhances gap junction coupling via its cell adhesion function and interaction with connexin 50.J Cell Sci. 2011 Jan 15;124(Pt 2):198-206. doi: 10.1242/jcs.072652. Epub 2010 Dec 20. J Cell Sci. 2011. PMID: 21172802 Free PMC article.
-
Selective interactions among the multiple connexin proteins expressed in the vertebrate lens: the second extracellular domain is a determinant of compatibility between connexins.J Cell Biol. 1994 May;125(4):879-92. doi: 10.1083/jcb.125.4.879. J Cell Biol. 1994. PMID: 8188753 Free PMC article.
-
Roles and regulation of lens epithelial cell connexins.FEBS Lett. 2014 Apr 17;588(8):1297-303. doi: 10.1016/j.febslet.2013.12.024. Epub 2014 Jan 14. FEBS Lett. 2014. PMID: 24434541 Free PMC article. Review.
-
Gap junctions or hemichannel-dependent and independent roles of connexins in cataractogenesis and lens development.Curr Mol Med. 2010 Dec;10(9):851-63. doi: 10.2174/156652410793937750. Curr Mol Med. 2010. PMID: 21091421 Free PMC article. Review.
Cited by
-
Beyond the Channels: Adhesion Functions of Aquaporin 0 and Connexin 50 in Lens Development.Front Cell Dev Biol. 2022 Apr 7;10:866980. doi: 10.3389/fcell.2022.866980. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35465319 Free PMC article. Review.
-
Arvcf Dependent Adherens Junction Stability is Required to Prevent Age-Related Cortical Cataracts.Front Cell Dev Biol. 2022 Jul 6;10:840129. doi: 10.3389/fcell.2022.840129. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35874813 Free PMC article.
-
Calcium-Activated Chloride Channels in Newly Differentiating Mouse Lens Fiber Cells and Their Role in Volume Regulation.Invest Ophthalmol Vis Sci. 2019 Apr 1;60(5):1621-1629. doi: 10.1167/iovs.19-26626. Invest Ophthalmol Vis Sci. 2019. PMID: 30995319 Free PMC article.
-
Genetic association of GJA8 with long-segment Hirschsprung's disease in southern Chinese children.Transl Pediatr. 2024 Aug 31;13(8):1395-1405. doi: 10.21037/tp-24-153. Epub 2024 Aug 28. Transl Pediatr. 2024. PMID: 39263294 Free PMC article.
-
Aquaporins Have Regional Functions in Development of Refractive Index in the Zebrafish Eye Lens.Invest Ophthalmol Vis Sci. 2021 Mar 1;62(3):23. doi: 10.1167/iovs.62.3.23. Invest Ophthalmol Vis Sci. 2021. PMID: 33724295 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

