Novel function of pregnancy-associated plasma protein A: promotes endometrium receptivity by up-regulating N-fucosylation
- PMID: 28706275
- PMCID: PMC5509645
- DOI: 10.1038/s41598-017-04735-0
Novel function of pregnancy-associated plasma protein A: promotes endometrium receptivity by up-regulating N-fucosylation
Erratum in
-
Author Correction: Novel function of pregnancy-associated plasma protein A: promotes endometrium receptivity by up-regulating N-fucosylation.Sci Rep. 2020 Jul 15;10(1):11964. doi: 10.1038/s41598-020-69040-9. Sci Rep. 2020. PMID: 32665707 Free PMC article.
Abstract
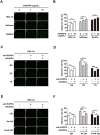
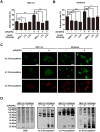
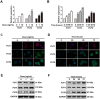
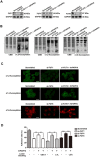
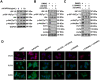
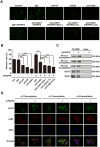
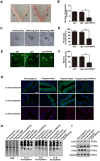
Glycosylation of uterine endometrial cells plays important roles to determine their receptive function to blastocysts. Trophoblast-derived pregnancy-associated plasma protein A (PAPPA) is specifically elevated in pregnant women serum, and is known to promote trophoblast cell proliferation and adhesion. However, the relationship between PAPPA and endometrium receptivity, as well as the regulation of N-fucosylation remains unclear. We found that rhPAPPA and PAPPA in the serum samples from pregnant women or conditioned medium of trophoblast cells promoted endometrium receptivity in vitro. Moreover, rhPAPPA increased α1,2-, α1,3- and α1,6-fucosylation levels by up-regulating N-fucosyltransferases FUT1, FUT4 and FUT8 expression, respectively, through IGF-1R/PI3K/Akt signaling pathway in human endometrial cells. Additionally, α1,2-, α1,3- and α1,6-fucosylation of integrin αVβ3, a critical endometrium receptivity biomarker, was up-regulated by PAPPA, thereby enhanced its adhesive functions. Furthermore, PAPPA blockage with antibody inhibited embryo implantation in vivo, mouse embryo adhesion and spreading in vitro, as well as N-fucosylation level of the endometrium in pregnant mice. In summary, this study suggests that PAPPA is essential to maintain a receptive endometrium by up-regulating N-fucosylation, which is a potential useful biomarker to evaluate the receptive functions of the endometrium.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
MicroRNA-200c impairs uterine receptivity formation by targeting FUT4 and α1,3-fucosylation.Cell Death Differ. 2017 Dec;24(12):2161-2172. doi: 10.1038/cdd.2017.136. Epub 2017 Sep 15. Cell Death Differ. 2017. PMID: 28914881 Free PMC article.
-
Administration of calcitonin promotes blastocyst implantation in mice by up-regulating integrin β3 expression in endometrial epithelial cells.Hum Reprod. 2012 Dec;27(12):3540-51. doi: 10.1093/humrep/des330. Epub 2012 Sep 20. Hum Reprod. 2012. PMID: 23001774
-
Differential expression of LeY and fucosyltransferase IV correlates with the receptivity of RL95-2 and HEC-1A human uterine epithelial cells.Cell Biol Int. 2012 May 1;36(5):469-74. doi: 10.1042/CBI20100644. Cell Biol Int. 2012. PMID: 22145955
-
Ion channels in the endometrium: regulation of endometrial receptivity and embryo implantation.Hum Reprod Update. 2014 Jul-Aug;20(4):517-29. doi: 10.1093/humupd/dmu006. Epub 2014 Mar 2. Hum Reprod Update. 2014. PMID: 24591147 Review.
-
The Sweet Relationship between the Endometrium and Protein Glycosylation.Biomolecules. 2024 Jun 27;14(7):770. doi: 10.3390/biom14070770. Biomolecules. 2024. PMID: 39062484 Free PMC article. Review.
Cited by
-
Pregnancy diagnosis and sex identification with urinary glycopatterns of two mammal species.iScience. 2023 Nov 14;26(12):108439. doi: 10.1016/j.isci.2023.108439. eCollection 2023 Dec 15. iScience. 2023. PMID: 38213790 Free PMC article.
-
Evolutionary divergence of embryo implantation in primates.Philos Trans R Soc Lond B Biol Sci. 2022 Dec 5;377(1865):20210256. doi: 10.1098/rstb.2021.0256. Epub 2022 Oct 17. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 36252209 Free PMC article. Review.
-
CBS and MAT2A improve methionine-mediated DNA synthesis through SAMTOR/mTORC1/S6K1/CAD pathway during embryo implantation.Cell Prolif. 2021 Jan;54(1):e12950. doi: 10.1111/cpr.12950. Epub 2020 Nov 12. Cell Prolif. 2021. PMID: 33179842 Free PMC article.
-
Improving pregnancy outcomes in patients with recurrent implantation failure: The power of RNA-seq-based endometrial receptivity testing.Medicine (Baltimore). 2024 Oct 25;103(43):e40210. doi: 10.1097/MD.0000000000040210. Medicine (Baltimore). 2024. PMID: 39470570 Free PMC article.
-
Vestibulodynia presentation is differentiated by the presence of additional chronic primary pain conditions.J Pain. 2025 Aug;33:105450. doi: 10.1016/j.jpain.2025.105450. Epub 2025 May 22. J Pain. 2025. PMID: 40412493
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

